Precision Medicine at the University of Alabama at Birmingham: Laying the Foundational Processes Through Implementation of Genotype-Guided Antiplatelet Therapy
- PMID: 28124392
- PMCID: PMC5526751
- DOI: 10.1002/cpt.631
Precision Medicine at the University of Alabama at Birmingham: Laying the Foundational Processes Through Implementation of Genotype-Guided Antiplatelet Therapy
Abstract
Precision medicine entails tailoring treatment based on patients' unique characteristics. As drug therapy constitutes the cornerstone of treatment for most chronic diseases, pharmacogenomics (PGx), the study of genetic variation influencing individual response to drugs, is an important component of precision medicine. Over the past decade investigations have identified genes and single-nucleotide polymorphisms (SNPs) and quantified their effect on drug response. Parallel development of point-of-care (POC) genotyping platforms has enabled the interrogation of the genes/SNPs within a timeline conducive to the provision of care. Despite these advances, the pace of integration of genotype-guided drug therapy (GGTx) into practice has faced significant challenges. These include difficulty in identifying SNPs with sufficiently robust evidence to guide clinical decision making, lack of clinician training on how to order and use genotype data, lack of clinical decision support (CDS) to guide treatment, and limited reimbursement. The University of Alabama at Birmingham's (UAB) efforts in precision medicine were initiated to address these challenges and improve the health of the racially diverse patients we treat.
© 2017 American Society for Clinical Pharmacology and Therapeutics.
Figures

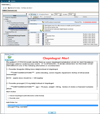
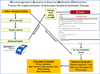
References
-
- Vladutiu GD. The FDA announces new drug labeling for pharmacogenetic testing: is personalized medicine becoming a reality? Mol Genet Metab. 2008;93:1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

