Effect of Sodium and Chloride Binding on a Lecithin Bilayer. A Molecular Dynamics Study
- PMID: 28125062
- PMCID: PMC5371966
- DOI: 10.3390/membranes7010005
Effect of Sodium and Chloride Binding on a Lecithin Bilayer. A Molecular Dynamics Study
Abstract
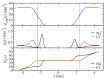
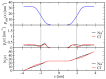
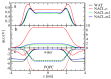
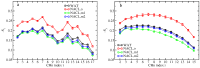
The effect of ion binding on the structural, mechanical, dynamic and electrostatic properties of a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayer in a 0.5 M aqueous NaCl solution is investigated using classical atomistic molecular dynamics simulation with different force-field descriptions for ion-ion and ion-lipid interactions. Most importantly, the repulsive Lennard-Jones parameters for the latter were modified, such that approximately similar binding of cations and anions to the lipid membrane is achieved. This was done to qualitatively improve the apparent ion-lipid binding constants obtained from simulations with the original force field (Berger lipids and GROMOS87 ions in combination with the SPC water model) in comparison to experimental data. Furthermore, various parameters characterizing membrane structure, elasticity, order and dynamics are analyzed. It is found that ion binding as observed in simulations involving the modified in comparison to the original force-field description leads to: (i) a smaller salt-induced change in the area per lipid, which is in closer agreement with the experiment; (ii) a decrease in the area compressibility and bilayer thickness to values comparable to a bilayer in pure water; (iii) lipid deuterium order parameters and lipid diffusion coefficients on nanosecond timescales that are very similar to the values for a membrane in pure water. In general, salt effects on the structural properties of a POPC bilayer in an aqueous sodium-chloride solution appear to be reproduced reasonably well by the new force-field description. An analysis of membrane-membrane disjoining pressure suggests that the smaller salt-induced change in area per lipid induced by the new force-field description is not due to the alteration of membrane-associated net charge, but must rather be understood as a consequence of ion-specific effects on the arrangement of lipid molecules.
Keywords: POPC bilayer; ion force field; lipid force field; molecular dynamics; salt effects; sodium chloride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lösche M., Möhwald H. Electrostatic interactions in phospholipid membranes. II. Influence of divalent ions on monolayer structure. J. Colloid Interface Sci. 1989;131:56–67. doi: 10.1016/0021-9797(89)90145-8. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

