Epstein-Barr Virus-induced Gene 2 Mediates Allergen-induced Leukocyte Migration into Airways
- PMID: 28125291
- PMCID: PMC5476910
- DOI: 10.1164/rccm.201608-1580OC
Epstein-Barr Virus-induced Gene 2 Mediates Allergen-induced Leukocyte Migration into Airways
Abstract
Rationale: Leukocyte recruitment to sites of allergic inflammation depends on the local production of priming cytokines, chemokines, and potentially other mediators. Previously, we showed that eosinophils (Eos) express numerous orphan G-protein-coupled receptors, including Epstein-Barr virus-induced gene 2 (EBI2). Despite its contribution to inflammatory diseases, the role of EBI2 in pulmonary eosinophilia is unknown.
Objectives: To determine whether oxysterol ligands for EBI2 are increased in asthma exacerbation, and if or how they promote Eos pulmonary migration.
Methods: EBI2 ligands and pulmonary eosinophilia were measured in the bronchoalveolar lavage fluid from patients with mild asthma 48 hours after acute allergen challenge. In vitro, the ability of EBI2 ligands alone or in combination with IL-5 priming to induce the migration of human blood Eos was assessed.
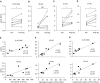
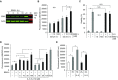
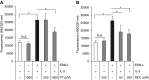
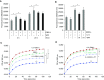
Measurements and main results: EBI2 was constitutively and stably expressed in peripheral blood Eos. Eos treated with the EBI2 ligands showed significantly increased transwell migration that was enhanced by priming with physiologic doses of IL-5. Migration was suppressed by inhibitors of the prolyl isomerase Pin1 or extracellular signal-regulated kinases (ERK) 1/2 or by pertussis toxin. EBI2 signaling activated Pin1 isomerase activity through a cascade that was sensitive to ERK inhibitors and pertussis toxin. The concentration of EBI2 ligands was significantly increased in the bronchoalveolar lavage fluid 48 hours after segmental allergen challenge and strongly correlated with the increased numbers of Eos, lymphocytes, and neutrophils in the airways.
Conclusions: Oxysterols are increased in inflamed airways after allergen challenge and, through G-protein subunit α, ERK, and Pin1 signaling, likely participate in the regulation of Eos migration into the lung in people with asthma.
Keywords: EBI2; Pin1; asthma; eosinophils; migration.
Figures

Comment in
-
Epstein-Barr Virus-induced Gene 2 and Leukocyte Airway Recruitment in Response to Allergen Challenge.Am J Respir Crit Care Med. 2017 Jun 15;195(12):1543-1544. doi: 10.1164/rccm.201701-0096ED. Am J Respir Crit Care Med. 2017. PMID: 28617082 No abstract available.
References
-
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. - PubMed
-
- Gauvreau GM, Boulet LP, Cockcroft DW, Baatjes A, Cote J, Deschesnes F, Davis B, Strinich T, Howie K, Duong M, et al. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med. 2008;177:952–958. - PubMed
-
- Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, Sousa AR, Martin J, Dallow N, Gilbert J, Allen A, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014;44:508–516. - PubMed
-
- Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: decreased CCR3 and increased CXCR4 expression by lung eosinophils. J Allergy Clin Immunol. 2001;108:563–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

