A Recombinant Human Anti-Platelet scFv Antibody Produced in Pichia pastoris for Atheroma Targeting
- PMID: 28125612
- PMCID: PMC5268420
- DOI: 10.1371/journal.pone.0170305
A Recombinant Human Anti-Platelet scFv Antibody Produced in Pichia pastoris for Atheroma Targeting
Abstract
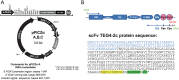
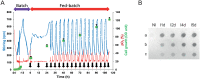
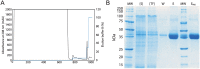
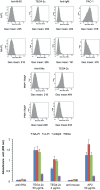
Cells of the innate and adaptive immune system are key factors in the progression of atherosclerotic plaque, leading to plaque instability and rupture, potentially resulting in acute atherothrombotic events such as coronary artery disease, cerebrovascular disease and peripheral arterial disease. Here, we describe the cloning, expression, purification, and immunoreactivity assessment of a recombinant single-chain variable fragment (scFv) derived from a human anti-αIIbβ3 antibody (HuAb) selected to target atheromatous lesions for the presence of platelets. Indeed, platelets within atheroma plaques have been shown to play a role in inflammation, in platelet-leucocyte aggregates and in thrombi formation and might thus be considered relevant biomarkers of atherosclerotic progression. The DNA sequence that encodes the anti-αIIbβ3 TEG4 scFv previously obtained from a phage-display selection on activated platelets, was inserted into the eukaryote vector (pPICZαA) in fusion with a tag sequence encoding 2 cysteines useable for specific probes grafting experiments. The recombinant protein was expressed at high yields in Pichia pastoris (30 mg/L culture). The advantage of P. pastoris as an expression system is the production and secretion of recombinant proteins in the supernatant, ruling out the difficulties encountered when scFv are produced in the cytoplasm of bacteria (low yield, low solubility and reduced affinity). The improved conditions allowed for the recovery of highly purified and biologically active scFv fragments ready to be grafted in a site-directed way to nanoparticles for the imaging of atherosclerotic plaques involving inflammatory processes and thus at high risk of instability.
Conflict of interest statement
Pall Life Sciences provided support on the Octet Red experiments. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
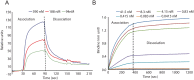
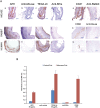
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

