NFATc3 and VIP in Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease
- PMID: 28125639
- PMCID: PMC5270325
- DOI: 10.1371/journal.pone.0170606
NFATc3 and VIP in Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease
Abstract
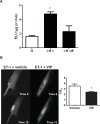
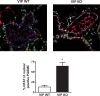
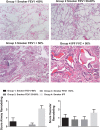
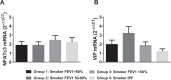
Idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) are both debilitating lung diseases which can lead to hypoxemia and pulmonary hypertension (PH). Nuclear Factor of Activated T-cells (NFAT) is a transcription factor implicated in the etiology of vascular remodeling in hypoxic PH. We have previously shown that mice lacking the ability to generate Vasoactive Intestinal Peptide (VIP) develop spontaneous PH, pulmonary arterial remodeling and lung inflammation. Inhibition of NFAT attenuated PH in these mice suggesting a connection between NFAT and VIP. To test the hypotheses that: 1) VIP inhibits NFAT isoform c3 (NFATc3) activity in pulmonary vascular smooth muscle cells; 2) lung NFATc3 activation is associated with disease severity in IPF and COPD patients, and 3) VIP and NFATc3 expression correlate in lung tissue from IPF and COPD patients. NFAT activity was determined in isolated pulmonary arteries from NFAT-luciferase reporter mice. The % of nuclei with NFAT nuclear accumulation was determined in primary human pulmonary artery smooth muscle cell (PASMC) cultures; in lung airway epithelia and smooth muscle and pulmonary endothelia and smooth muscle from IPF and COPD patients; and in PASMC from mouse lung sections by fluorescence microscopy. Both NFAT and VIP mRNA levels were measured in lungs from IPF and COPD patients. Empirical strategies applied to test hypotheses regarding VIP, NFATc3 expression and activity, and disease type and severity. This study shows a significant negative correlation between NFAT isoform c3 protein expression levels in PASMC, activity of NFATc3 in pulmonary endothelial cells, expression and activity of NFATc3 in bronchial epithelial cells and lung function in IPF patients, supporting the concept that NFATc3 is activated in the early stages of IPF. We further show that there is a significant positive correlation between NFATc3 mRNA expression and VIP RNA expression only in lungs from IPF patients. In addition, we found that VIP inhibits NFAT nuclear translocation in primary human pulmonary artery smooth muscle cells (PASMC). Early activation of NFATc3 in IPF patients may contribute to disease progression and the increase in VIP expression could be a protective compensatory mechanism.
Conflict of interest statement
Three Village Allergy & Asthma, PLLC provided support in the form of salaries for A.M.S. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
PDZ-Binding Kinase, a Novel Regulator of Vascular Remodeling in Pulmonary Arterial Hypertension.Circulation. 2024 Jul 30;150(5):393-410. doi: 10.1161/CIRCULATIONAHA.123.067095. Epub 2024 Apr 29. Circulation. 2024. PMID: 38682326 Free PMC article.
-
LncRNA-536 and RNA-Binding Protein RBM25 Interactions in Pulmonary Artery Smooth Muscle Cells: Implications in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2025 Aug;45(8):e374-e391. doi: 10.1161/ATVBAHA.125.322734. Epub 2025 Jun 26. Arterioscler Thromb Vasc Biol. 2025. PMID: 40567228
-
RhoA increases ASIC1a plasma membrane localization and calcium influx in pulmonary arterial smooth muscle cells following chronic hypoxia.Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C166-C176. doi: 10.1152/ajpcell.00159.2017. Epub 2017 Oct 25. Am J Physiol Cell Physiol. 2018. PMID: 29070491 Free PMC article.
-
Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Jul 20;7(7):CD013196. doi: 10.1002/14651858.CD013196.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693988 Free PMC article.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
Cited by
-
eNAMPT Is a Novel Damage-associated Molecular Pattern Protein That Contributes to the Severity of Radiation-induced Lung Fibrosis.Am J Respir Cell Mol Biol. 2022 May;66(5):497-509. doi: 10.1165/rcmb.2021-0357OC. Am J Respir Cell Mol Biol. 2022. PMID: 35167418 Free PMC article.
-
Neuroimmune Interaction: A Widespread Mutual Regulation and the Weapons for Barrier Organs.Front Cell Dev Biol. 2022 May 11;10:906755. doi: 10.3389/fcell.2022.906755. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646918 Free PMC article. Review.
-
The Neuropeptide VIP Limits Human Osteoclastogenesis: Clinical Associations with Bone Metabolism Markers in Patients with Early Arthritis.Biomedicines. 2021 Dec 10;9(12):1880. doi: 10.3390/biomedicines9121880. Biomedicines. 2021. PMID: 34944693 Free PMC article.
-
Mechanistic Insights and Therapeutic Potential of Wnt5a Signaling in Alveolar Epithelial Cell Development and Bronchopulmonary Dysplasia.Stem Cell Rev Rep. 2025 Aug 16. doi: 10.1007/s12015-025-10951-3. Online ahead of print. Stem Cell Rev Rep. 2025. PMID: 40817998 Review.
-
lncRNA NEAT1 promotes hypoxia-induced inflammation and fibrosis of alveolar epithelial cells via targeting miR-29a/NFATc3 axis.Kaohsiung J Med Sci. 2022 Aug;38(8):739-748. doi: 10.1002/kjm2.12535. Epub 2022 Jun 16. Kaohsiung J Med Sci. 2022. PMID: 35708150 Free PMC article.
References
-
- Adir Y, Harari S (2014) Pulmonary hypertension associated with chronic obstructive lung disease and idiopathic pulmonary fibrosis. Current Opinion in Pulmonary Medicine 20. - PubMed
-
- Bierer R, Nitta CH, Friedman JK, Codianni SJ, De Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Gonzalez Bosc LV (2011) NFATc3 Is Required for Chronic Hypoxia-induced Pulmonary Hypertension in Adult and Neonatal Mice. American Journal of Physiology—Lung Cellular and Molecular Physiology. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

