Development of a Novel Vaccine Containing Binary Toxin for the Prevention of Clostridium difficile Disease with Enhanced Efficacy against NAP1 Strains
- PMID: 28125650
- PMCID: PMC5268477
- DOI: 10.1371/journal.pone.0170640
Development of a Novel Vaccine Containing Binary Toxin for the Prevention of Clostridium difficile Disease with Enhanced Efficacy against NAP1 Strains
Abstract
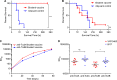
Clostridium difficile infections (CDI) are a leading cause of nosocomial diarrhea in the developed world. The main virulence factors of the bacterium are the large clostridial toxins (LCTs), TcdA and TcdB, which are largely responsible for the symptoms of the disease. Recent outbreaks of CDI have been associated with the emergence of hypervirulent strains, such as NAP1/BI/027, many strains of which also produce a third toxin, binary toxin (CDTa and CDTb). These hypervirulent strains have been associated with increased morbidity and higher mortality. Here we present pre-clinical data describing a novel tetravalent vaccine composed of attenuated forms of TcdA, TcdB and binary toxin components CDTa and CDTb. We demonstrate, using the Syrian golden hamster model of CDI, that the inclusion of binary toxin components CDTa and CDTb significantly improves the efficacy of the vaccine against challenge with NAP1 strains in comparison to vaccines containing only TcdA and TcdB antigens, while providing comparable efficacy against challenge with the prototypic, non-epidemic strain VPI10463. This combination vaccine elicits high neutralizing antibody titers against TcdA, TcdB and binary toxin in both hamsters and rhesus macaques. Finally we present data that binary toxin alone can act as a virulence factor in animal models. Taken together, these data strongly support the inclusion of binary toxin in a vaccine against CDI to provide enhanced protection from epidemic strains of C. difficile.
Conflict of interest statement
SS, SW, JD, JX, RRR, MH, RX, BW, CL, AK, SCW, SC, SV, MPG, AG, JS, ES, DST, JLB, and JHH are current or past employees of Merck as stated in the affiliations and potentially own stock and/or hold stock options in the company. Other authors have no competing interests. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures



References
-
- Cohen S, Gerding DN, Johnson S, Kelly C, Loo V, McDonald C, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. 10.1086/651706 - DOI - PubMed
-
- Miller B, Chen L, Sexton D, Anderson D . Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387–90. - PubMed
-
- Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. The Lancet. 2005;366(9491):1079–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

