Lack of Casein Kinase 1 Delta Promotes Genomic Instability - The Accumulation of DNA Damage and Down-Regulation of Checkpoint Kinase 1
- PMID: 28125685
- PMCID: PMC5268481
- DOI: 10.1371/journal.pone.0170903
Lack of Casein Kinase 1 Delta Promotes Genomic Instability - The Accumulation of DNA Damage and Down-Regulation of Checkpoint Kinase 1
Abstract
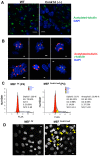
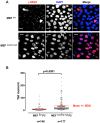
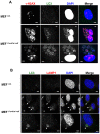
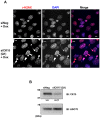
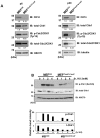
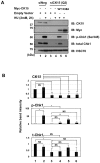
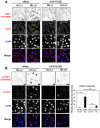
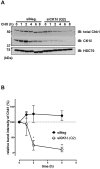
Casein kinase 1 delta (CK1δ) is a conserved serine/threonine protein kinase that regulates diverse cellular processes. Mice lacking CK1δ have a perinatal lethal phenotype and typically weigh 30% less than their wild type littermates. However, the causes of death and small size are unknown. We observed cells with abnormally large nuclei in tissue from Csnk1d null embryos, and multiple centrosomes in mouse embryo fibroblasts (MEFs) deficient in CK1δ (MEFCsnk1d null). Results from γ-H2AX staining and the comet assay demonstrated significant DNA damage in MEFCsnk1d null cells. These cells often contain micronuclei, an indicator of genomic instability. Similarly, abrogation of CK1δ expression in control MEFs stimulated micronuclei formation after doxorubicin treatment, suggesting that CK1δ loss increases vulnerability to genotoxic stress. Cellular levels of total and activated checkpoint kinase 1 (Chk1), which functions in the DNA damage response and mitotic checkpoints, and its downstream effector, Cdc2/CDK1 kinase, were often decreased in MEFCsnk1d null cells as well as in control MEFs transfected with CK1δ siRNA. Hydroxyurea-induced Chk1 activation, as measured by Ser345 phosphorylation, and nuclear localization also were impaired in MEF cells following siRNA knockdown of CK1δ. Similar results were observed in the MCF7 human breast cancer cell line. The decreases in phosphorylated Chk1 were rescued by concomitant expression of siRNA-resistant CK1δ. Experiments with cycloheximide demonstrated that the stability of Chk1 protein was diminished in cells subjected to CK1δ knockdown. Together, these findings suggest that CK1δ contributes to the efficient repair of DNA damage and the proper functioning of mitotic checkpoints by maintaining appropriate levels of Chk1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10(10):699–711. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

