Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study
- PMID: 28125977
- PMCID: PMC5270253
- DOI: 10.1186/s12877-017-0426-3
Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study
Abstract
Background: In the nursing home population, it is estimated that 1 in every 3 patients is polymedicated and given their considerable frailty, these patients are especially prone to adverse drug reactions. Clinical pharmacist-led medication reviews are considered successful interventions to improve medication safety in the inpatient setting. Due to the limited available evidence concerning the benefits of medication reviews performed in the nursing home setting, we propose a study aiming to demonstrate a positive effect that a clinical decision support system, as a health care intervention, may have on the target population. The primary objective of this study is to reduce the number of patients with at least one event when using the clinical decision support system compared to the regular care. These events consist of hospital referrals, delirium, falls, and/or deaths.
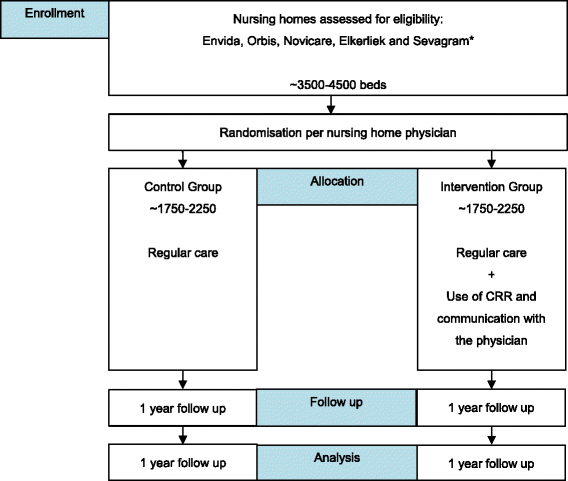
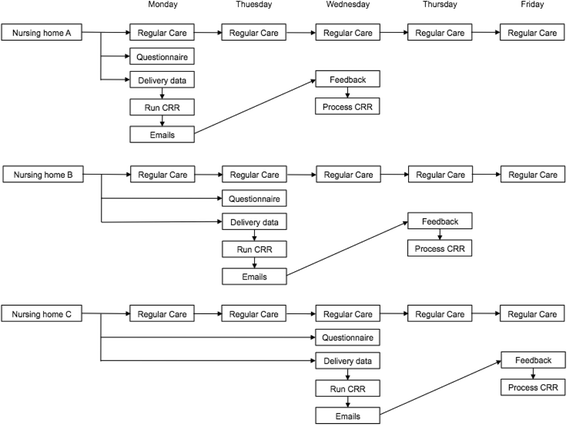
Method/design: This study is a multicentre, prospective, randomised study with a cluster group design. The randomisation will be per main nursing home physician and stratified per ward (somatic and psychogeriatric). In the intervention group the clinical decision support system will be used to screen medication list, laboratory values and medical history in order to obtain potential clinical relevant remarks. The remarks will be sent to the main physician and feedback will be provided whether the advice was followed or not. In the control group regular care will be applied.
Discussion: We strongly believe that by using a clinical decision support system, medication reviews are performed in a standardised way which leads to comparable results between patients. In addition, using a clinical decision support system eliminates the time factor to perform medication reviews as the major problems related to medication, laboratory values, indications and/or established patient characteristics will be directly available. In this way, and in order to make the medication review process complete, consultation within healthcare professionals and/or the patient itself will be time effective and the medication surveillance could be performed around the clock.
Trial registration: The Netherlands National Trial Register NTR5165 . Registered 2nd April 2015.
Keywords: Aged; Decision support systems management; Medication review; Medication therapy management; Polypharmacy.
Figures
References
-
- Multidisciplinaire Richtlijn Polyfarmacie bij ouderen 2012. https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarm.... Accessed Nov 2014.
-
- https://www.sfk.nl/nieuws-publicaties/PW/2012/polyfarmacie-voor-1-op-de-.... Accessed 30 Jul 2013.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous