The correlation between the length of repetitive domain and mechanical properties of the recombinant flagelliform spidroin
- PMID: 28126711
- PMCID: PMC5374401
- DOI: 10.1242/bio.022665
The correlation between the length of repetitive domain and mechanical properties of the recombinant flagelliform spidroin
Abstract
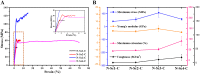
Spider silk is an attractive biopolymer with numerous potential applications due to its remarkable characteristics. Among the six categories of spider silks, flagelliform (Flag) spider silk possesses longer and more repetitive core domains than others, therefore performing the highest extensibility. To investigate the correlation between the recombinant spidroin size and the synthetic fiber properties, four recombinant proteins with different sizes [N-Scn-C (n=1-4)] were constructed and expressed using IMPACT system. Subsequently, different recombinant spidroins were spun into fibers through wet-spinning via a custom-made continuous post-drawing device. Mechanical tests of the synthetic fibers with four parameters (maximum stress, maximum extension, Young's modulus and toughness) demonstrated that the extensibility of the fibers showed a positive correlation with spidroin size, consequently resulting in the extensibility of N-Sc4-C fiber ranked the highest (58.76%) among four fibers. Raman data revealed the relationship between secondary structure content and mechanical properties. The data here provide a deeper insight into the relationship between the function and structure of Flag silk for future design of artificial fibers.
Keywords: Fiber structure; Flagelliform silk; Mechanical properties; Synthetic fiber; Wet-spinning.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





Similar articles
-
Conserved C-terminal domain of spider tubuliform spidroin 1 contributes to extensibility in synthetic fibers.Biomacromolecules. 2012 Feb 13;13(2):304-12. doi: 10.1021/bm201262n. Epub 2012 Jan 12. Biomacromolecules. 2012. PMID: 22176138
-
Characterization of the second type of aciniform spidroin (AcSp2) provides new insight into design for spidroin-based biomaterials.Acta Biomater. 2020 Oct 1;115:210-219. doi: 10.1016/j.actbio.2020.08.009. Epub 2020 Aug 13. Acta Biomater. 2020. PMID: 32798722
-
Tensile properties of synthetic pyriform spider silk fibers depend on the number of repetitive units as well as the presence of N- and C-terminal domains.Int J Biol Macromol. 2020 Jul 1;154:765-772. doi: 10.1016/j.ijbiomac.2020.03.042. Epub 2020 Mar 10. Int J Biol Macromol. 2020. PMID: 32169447
-
Strategies for Making High-Performance Artificial Spider Silk Fibers.Adv Funct Mater. 2024 Aug 28;34(35):2305040. doi: 10.1002/adfm.202305040. Epub 2023 Oct 10. Adv Funct Mater. 2024. PMID: 39355086 Free PMC article. Review.
-
Controlled assembly: a prerequisite for the use of recombinant spider silk in regenerative medicine?Acta Biomater. 2014 Apr;10(4):1627-31. doi: 10.1016/j.actbio.2013.09.030. Epub 2013 Oct 1. Acta Biomater. 2014. PMID: 24090990 Review.
Cited by
-
Egg Case Silk Gene Sequences from Argiope Spiders: Evidence for Multiple Loci and a Loss of Function Between Paralogs.G3 (Bethesda). 2018 Jan 4;8(1):231-238. doi: 10.1534/g3.117.300283. G3 (Bethesda). 2018. PMID: 29127108 Free PMC article.
-
Chromosome-level genome and the identification of sex chromosomes in Uloborus diversus.Gigascience. 2022 Dec 28;12:giad002. doi: 10.1093/gigascience/giad002. Epub 2023 Feb 10. Gigascience. 2022. PMID: 36762707 Free PMC article.
-
Spidroin Silk Fibers with Bioactive Motifs of Extracellular Proteins for Neural Tissue Engineering.ACS Omega. 2021 May 30;6(23):15264-15273. doi: 10.1021/acsomega.1c01576. eCollection 2021 Jun 15. ACS Omega. 2021. PMID: 34151105 Free PMC article.
-
Influence of Spider Silk Protein Structure on Mechanical and Biological Properties for Energetic Material Detection.Molecules. 2024 Feb 27;29(5):1025. doi: 10.3390/molecules29051025. Molecules. 2024. PMID: 38474537 Free PMC article. Review.
-
Customized Flagelliform Spidroins Form Spider Silk-like Fibers at pH 8.0 with Outstanding Tensile Strength.ACS Biomater Sci Eng. 2022 Jan 10;8(1):119-127. doi: 10.1021/acsbiomaterials.1c01354. Epub 2021 Dec 15. ACS Biomater Sci Eng. 2022. PMID: 34908395 Free PMC article.
References
-
- Adrianos S. L., Teulé F., Hinman M. B., Jones J. A., Weber W. S., Yarger J. L. and Lewis R. V. (2013). Nephila clavipes Flagelliform silk-like GGX motifs contribute to extensibility and spacer motifs contribute to strength in synthetic spider silk fibers. Biomacromolecules 14, 1751-1760. 10.1021/bm400125w - DOI - PMC - PubMed
-
- Andersson M., Chen G., Otikovs M., Landreh M., Nordling K., Kronqvist N., Westermark P., Jörnvall H., Knight S., Ridderstråle Y. et al. (2014). Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains. PLoS Biol. 12, e1001921 10.1371/journal.pbio.1001921 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

