Design principles for robust vesiculation in clathrin-mediated endocytosis
- PMID: 28126722
- PMCID: PMC5320970
- DOI: 10.1073/pnas.1617705114
Design principles for robust vesiculation in clathrin-mediated endocytosis
Abstract
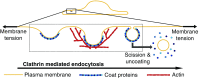
A critical step in cellular-trafficking pathways is the budding of membranes by protein coats, which recent experiments have demonstrated can be inhibited by elevated membrane tension. The robustness of processes like clathrin-mediated endocytosis (CME) across a diverse range of organisms and mechanical environments suggests that the protein machinery in this process has evolved to take advantage of some set of physical design principles to ensure robust vesiculation against opposing forces like membrane tension. Using a theoretical model for membrane mechanics and membrane protein interaction, we have systematically investigated the influence of membrane rigidity, curvature induced by the protein coat, area covered by the protein coat, membrane tension, and force from actin polymerization on bud formation. Under low tension, the membrane smoothly evolves from a flat to budded morphology as the coat area or spontaneous curvature increases, whereas the membrane remains essentially flat at high tensions. At intermediate, physiologically relevant, tensions, the membrane undergoes a "snap-through instability" in which small changes in the coat area, spontaneous curvature or membrane tension cause the membrane to "snap" from an open, U-shape to a closed bud. This instability can be smoothed out by increasing the bending rigidity of the coat, allowing for successful budding at higher membrane tensions. Additionally, applied force from actin polymerization can bypass the instability by inducing a smooth transition from an open to a closed bud. Finally, a combination of increased coat rigidity and force from actin polymerization enables robust vesiculation even at high membrane tensions.
Keywords: clathrin-mediated endocytosis; membrane modeling; membrane tension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Endocytosis: clathrin-mediated membrane budding.Curr Opin Cell Biol. 2007 Aug;19(4):417-25. doi: 10.1016/j.ceb.2007.05.003. Epub 2007 Jul 13. Curr Opin Cell Biol. 2007. PMID: 17631994 Review.
-
Endocytic proteins drive vesicle growth via instability in high membrane tension environment.Proc Natl Acad Sci U S A. 2015 Mar 24;112(12):E1423-32. doi: 10.1073/pnas.1418491112. Epub 2015 Mar 9. Proc Natl Acad Sci U S A. 2015. PMID: 25775509 Free PMC article.
-
Minimal mesoscale model for protein-mediated vesiculation in clathrin-dependent endocytosis.PLoS Comput Biol. 2010 Sep 9;6(9):e1000926. doi: 10.1371/journal.pcbi.1000926. PLoS Comput Biol. 2010. PMID: 20838575 Free PMC article.
-
Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis.Biol Cell. 2021 Aug;113(8):344-373. doi: 10.1111/boc.202000110. Epub 2021 Apr 28. Biol Cell. 2021. PMID: 33788963 Free PMC article. Review.
-
Mechanoregulation of clathrin-mediated endocytosis.J Cell Sci. 2017 Nov 1;130(21):3631-3636. doi: 10.1242/jcs.205930. Epub 2017 Sep 18. J Cell Sci. 2017. PMID: 28923837 Free PMC article.
Cited by
-
Spatial regulation of clathrin-mediated endocytosis through position-dependent site maturation.J Cell Biol. 2020 Nov 2;219(11):e202002160. doi: 10.1083/jcb.202002160. J Cell Biol. 2020. PMID: 33053166 Free PMC article.
-
Nonaxisymmetric Shapes of Biological Membranes from Locally Induced Curvature.Biophys J. 2020 Sep 15;119(6):1065-1077. doi: 10.1016/j.bpj.2020.07.021. Epub 2020 Jul 31. Biophys J. 2020. PMID: 32860742 Free PMC article.
-
Geometric coupling of helicoidal ramps and curvature-inducing proteins in organelle membranes.J R Soc Interface. 2019 Sep 27;16(158):20190354. doi: 10.1098/rsif.2019.0354. Epub 2019 Sep 4. J R Soc Interface. 2019. PMID: 31480932 Free PMC article.
-
Membrane free-energy landscapes derived from atomistic dynamics explain nonuniversal cholesterol-induced stiffening.bioRxiv [Preprint]. 2023 Feb 17:2023.02.02.525347. doi: 10.1101/2023.02.02.525347. bioRxiv. 2023. Update in: PNAS Nexus. 2023 Aug 17;2(8):pgad269. doi: 10.1093/pnasnexus/pgad269. PMID: 36778237 Free PMC article. Updated. Preprint.
-
Lipid self-assembly and lectin-induced reorganization of the plasma membrane.Philos Trans R Soc Lond B Biol Sci. 2018 May 26;373(1747):20170117. doi: 10.1098/rstb.2017.0117. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29632269 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

