Loss-less Nano-fractionator for High Sensitivity, High Coverage Proteomics
- PMID: 28126900
- PMCID: PMC5383787
- DOI: 10.1074/mcp.O116.065136
Loss-less Nano-fractionator for High Sensitivity, High Coverage Proteomics
Abstract
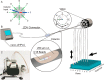
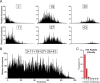
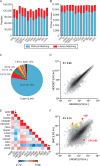
Recent advances in mass spectrometry (MS)-based proteomics now allow very deep coverage of cellular proteomes. To achieve near-comprehensive identification and quantification, the combination of a first HPLC-based peptide fractionation orthogonal to the on-line LC-MS/MS step has proven to be particularly powerful. This first dimension is typically performed with milliliter/min flow and relatively large column inner diameters, which allow efficient pre-fractionation but typically require peptide amounts in the milligram range. Here, we describe a novel approach termed "spider fractionator" in which the post-column flow of a nanobore chromatography system enters an eight-port flow-selector rotor valve. The valve switches the flow into different flow channels at constant time intervals, such as every 90 s. Each flow channel collects the fractions into autosampler vials of the LC-MS/MS system. Employing a freely configurable collection mechanism, samples are concatenated in a loss-less manner into 2-96 fractions, with efficient peak separation. The combination of eight fractions with 100 min gradients yields very deep coverage at reasonable measurement time, and other parameters can be chosen for even more rapid or for extremely deep measurements. We demonstrate excellent sensitivity by decreasing sample amounts from 100 μg into the sub-microgram range, without losses attributable to the spider fractionator and while quantifying close to 10,000 proteins. Finally, we apply the system to the rapid automated and in-depth characterization of 12 different human cell lines to a median depth of 11,472 different proteins, which revealed differences recapitulating their developmental origin and differentiation status. The fractionation technology described here is flexible, easy to use, and facilitates comprehensive proteome characterization with minimal sample requirements.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The other authors have no conflicts of interest
Figures


References
-
- Beck M., Claassen M., and Aebersold R. (2011) Comprehensive proteomics. Curr. Opin. Biotechnol. 22, 3–8 - PubMed
-
- Kulak N. A., Pichler G., Paron I., Nagaraj N., and Mann M. (2014) Minimal encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 11, 319–324 - PubMed
-
- Muñoz J., and Heck A. J. (2014) From the human genome to the human proteome. Angewandte Chemie 53, 10864–10866 - PubMed
-
- Aebersold R., and Mann M. (2016) Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

