Fibromodulin Expression in Folliculostellate Cells and Pericytes Is Promoted by TGFβ Signaling in Rat Anterior Pituitary Gland
- PMID: 28127105
- PMCID: PMC5263227
- DOI: 10.1267/ahc.16021
Fibromodulin Expression in Folliculostellate Cells and Pericytes Is Promoted by TGFβ Signaling in Rat Anterior Pituitary Gland
Abstract
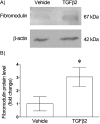
Fibromodulin belongs to the family of small leucine-rich proteoglycans (SLRPs), an active component of extracellular matrix. It directly binds collagens to promote fibrillogenesis and also binds transforming growth factor-beta (TGFβ) to antagonize its actions. Our previous studies of rat anterior pituitary gland revealed that fibromodulin is expressed in folliculostellate cells and pericytes. Although our recent study showed that TGFβ2 secreted from folliculostellate cells induces collagen synthesis in pericytes, the involvement of fibromodulin in TGFβ2-mediated collagen regulation has not been studied. The present study examined the effect of TGFβ2 on fibromodulin synthesis in rat anterior pituitary gland. In situ hybridization for TGFβ receptor II and immunohistological techniques revealed the presence of TGFβ receptor II in folliculostellate cells and pericytes. To confirm canonical TGFβ intracellular signaling, Smad2 immunocytochemistry was performed. Nuclear translocation of Smad2 was observed in folliculostellate cells and pericytes after TGFβ2 treatment. TGFβ2 strongly enhanced fibromodulin mRNA and protein expressions, and TGFβ2-induced mRNA expression was completely blocked by TGFβ receptor I inhibitor (SB431542). These results suggest that folliculostellate cells and pericytes exhibit canonical TGFβ2 signaling, which is associated with fibromodulin production. Thus, this is the first report to show that TGFβ signaling regulates the endogenous TGFβ antagonist fibromodulin in the gland.
Keywords: fibromodulin; folliculostellate cell; pericyte; small leucine-rich proteoglycans; transforming growth factor beta.
Figures



Similar articles
-
Folliculostellate cell interacts with pericyte via TGFβ2 in rat anterior pituitary.J Endocrinol. 2016 May;229(2):159-70. doi: 10.1530/JOE-16-0033. Epub 2016 Mar 8. J Endocrinol. 2016. PMID: 26957638
-
Laminin and collagen modulate expression of the small leucine-rich proteoglycan fibromodulin in rat anterior pituitary gland.Cell Tissue Res. 2013 Nov;354(2):633-8. doi: 10.1007/s00441-013-1698-3. Cell Tissue Res. 2013. PMID: 23959432
-
TGFβ signaling reinforces pericyte properties of the non-endocrine mouse pituitary cell line TtT/GF.Cell Tissue Res. 2018 Feb;371(2):339-350. doi: 10.1007/s00441-017-2758-x. Epub 2017 Dec 22. Cell Tissue Res. 2018. PMID: 29274061
-
Proteoglycans and diseases of soft tissues.Adv Exp Med Biol. 2014;802:49-58. doi: 10.1007/978-94-007-7893-1_4. Adv Exp Med Biol. 2014. PMID: 24443020 Review.
-
Functions of lumican and fibromodulin: lessons from knockout mice.Glycoconj J. 2002 May-Jun;19(4-5):287-93. doi: 10.1023/A:1025348417078. Glycoconj J. 2002. PMID: 12975607 Review.
Cited by
-
Role of Cancer Stem-like Cells in the Process of Invasion and Mesenchymal Transformation by a Reconstituted Triple-negative Breast Cancer Cell Population Resistant to p53-induced Apoptosis.Acta Histochem Cytochem. 2022 Oct 28;55(5):169-184. doi: 10.1267/ahc.22-00076. Epub 2022 Oct 25. Acta Histochem Cytochem. 2022. PMID: 36405550 Free PMC article.
-
Stem-like Human Breast Cancer Cells Initiate Vasculogenic Mimicry on Matrigel.Acta Histochem Cytochem. 2018 Dec 20;51(6):173-183. doi: 10.1267/ahc.18041. Epub 2018 Dec 15. Acta Histochem Cytochem. 2018. PMID: 30647492 Free PMC article.
-
Positive and negative feedback regulation of the TGF-β1 explains two equilibrium states in skin aging.iScience. 2024 Apr 10;27(5):109708. doi: 10.1016/j.isci.2024.109708. eCollection 2024 May 17. iScience. 2024. PMID: 38706856 Free PMC article.
References
-
- Allaerts W. and Vankelecom H. (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur. J. Endocrinol. 153; 1–12. - PubMed
-
- Burton-Wurster N., Liu W., Matthews G. L., Lust G., Roughley P. J., Glant T. T. and Cs-Szabo G. (2003) TGF beta 1 and biglycan, decorin, and fibromodulin metabolism in canine cartilage. Osteoarthritis Cartilage 11; 167–176. - PubMed
-
- Fujiwara K., Maekawa F., Kikuchi M., Takigami S., Yada T. and Yashiro T. (2007) Expression of retinaldehyde dehydrogenase (RALDH)2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res. 328; 129–135. - PubMed
-
- Fujiwara K., Jindatip D., Kikuchi M. and Yashiro T. (2010) In situ hybridization reveals that type I and III collagens are produced by pericytes in the anterior pituitary gland of rats. Cell Tissue Res. 342; 491–495. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

