Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas aeruginosa
- PMID: 28127379
- PMCID: PMC5239991
- DOI: 10.1155/2017/5163575
Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas aeruginosa
Abstract
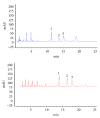
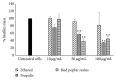
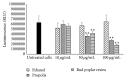
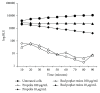
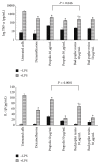
Pseudomonas aeruginosa is a common biofilm-forming bacterial pathogen implicated in lung, skin, and systemic infections. Biofilms are majorly associated with chronic lung infection, which is the most severe complication in cystic fibrosis patients characterized by drug-resistant biofilms in the bronchial mucus with zones, where reactive oxygen species concentration is increased mainly due to neutrophil activity. Aim of this work is to verify the anti-Pseudomonas property of propolis or bud poplar resins extracts. The antimicrobial activity of propolis and bud poplar resins extracts was determined by MIC and biofilm quantification. Moreover, we tested the antioxidant activity by DPPH and neutrophil oxidative burst assays. In the end, both propolis and bud poplar resins extracts were able to inhibit P. aeruginosa biofilm formation and to influence both swimming and swarming motility. Moreover, the extracts could inhibit proinflammatory cytokine production by human PBMC and showed both direct and indirect antioxidant activity. This work is the first to demonstrate that propolis and bud poplar resins extracts can influence biofilm formation of P. aeruginosa contrasting the inflammation and the oxidation state typical of chronic infection suggesting that propolis or bud poplar resins can be used along with antibiotic as adjuvant in the therapy against P. aeruginosa infections related to biofilm.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Bankova V. S., De Castro S. L., Marcucci M. C. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. doi: 10.1051/apido:2000102. - DOI
-
- Bankova V., Popova M., Bogdanov S., Sabatini A.-G. Chemical composition of European propolis: expected and unexpected results. Zeitschrift für Naturforschung C: A Journal of Biosciences. 2002;57(5-6):530–533. - PubMed
-
- Dudonné S., Poupard P., Coutiére P., et al. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: individual antioxidant contribution of phenolics and transcriptional effect on skin aging. Journal of Agricultural and Food Chemistry. 2011;59(9):4527–4536. doi: 10.1021/jf104791t. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

