Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice
- PMID: 28127564
- PMCID: PMC5239976
- DOI: 10.1155/2017/1763292
Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice
Erratum in
-
Corrigendum to "Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice".J Diabetes Res. 2024 Jul 17;2024:9809651. doi: 10.1155/2024/9809651. eCollection 2024. J Diabetes Res. 2024. PMID: 39049958 Free PMC article.
Abstract
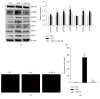
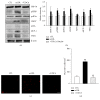
Loss of pericytes, an early hallmark of diabetic retinopathy (DR), results in breakdown of the blood-retinal barrier. Endoplasmic reticulum (ER) stress may be involved in this process. The purpose of this study was to examine the effects of ursodeoxycholic acid (UDCA), a known ameliorator of ER stress, on pericyte loss in DR of streptozotocin- (STZ-) induced diabetic mice. To assess the extent of DR, the integrity of retinal vessels and density of retinal capillaries in STZ-induced diabetic mice were evaluated. Additionally, induction of ER stress and the unfolded protein response (UPR) were assessed in diabetic mice and human retinal pericytes exposed to advanced glycation end products (AGE) or modified low-density lipoprotein (mLDL). Fluorescein dye leakage during angiography and retinal capillary density were improved in UDCA-treated diabetic mice, compared to the nontreated diabetic group. Among the UPR markers, those involved in the protein kinase-like ER kinase (PERK) pathway were increased, while UDCA attenuated UPR in STZ-induced diabetic mice as well as AGE- or mLDL-exposed retinal pericytes in culture. Consequently, vascular integrity was improved and pericyte loss reduced in the retina of STZ-induced diabetic mice. Our findings suggest that UDCA might be effective in protecting against DR.
Conflict of interest statement
The authors declare no competing interests relevant to this article.
Figures
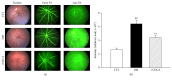
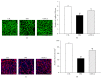
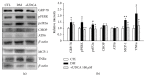
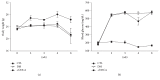
Similar articles
-
Ursodeoxycholic Acid Attenuates the Retinal Vascular Abnormalities in Anti-PDGFR-β Antibody-Induced Pericyte Depletion Mouse Models.Sci Rep. 2020 Jan 22;10(1):977. doi: 10.1038/s41598-020-58039-x. Sci Rep. 2020. PMID: 31969665 Free PMC article.
-
Ursodeoxycholic acid ameliorates diabetic retinopathy via reducing retinal inflammation and reversing the breakdown of blood-retinal barrier.Eur J Pharmacol. 2018 Dec 5;840:20-27. doi: 10.1016/j.ejphar.2018.09.027. Epub 2018 Sep 27. Eur J Pharmacol. 2018. PMID: 30268667
-
Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy.Exp Eye Res. 2020 Jun;195:108043. doi: 10.1016/j.exer.2020.108043. Epub 2020 May 4. Exp Eye Res. 2020. PMID: 32376470 Free PMC article.
-
Targeting pericyte retention in Diabetic Retinopathy: a review.Ann Med. 2024 Dec;56(1):2398200. doi: 10.1080/07853890.2024.2398200. Epub 2024 Sep 13. Ann Med. 2024. PMID: 39268600 Free PMC article. Review.
-
The unfolded protein response and diabetic retinopathy.J Diabetes Res. 2014;2014:160140. doi: 10.1155/2014/160140. Epub 2014 Oct 29. J Diabetes Res. 2014. PMID: 25530974 Free PMC article. Review.
Cited by
-
The role and mechanisms of gut microbiota in diabetic nephropathy, diabetic retinopathy and cardiovascular diseases.Front Microbiol. 2022 Aug 18;13:977187. doi: 10.3389/fmicb.2022.977187. eCollection 2022. Front Microbiol. 2022. PMID: 36060752 Free PMC article. Review.
-
Neurovascular Impairment and Therapeutic Strategies in Diabetic Retinopathy.Int J Environ Res Public Health. 2021 Dec 31;19(1):439. doi: 10.3390/ijerph19010439. Int J Environ Res Public Health. 2021. PMID: 35010703 Free PMC article. Review.
-
Research progress of diabetic retinopathy and gut microecology.Front Microbiol. 2023 Sep 7;14:1256878. doi: 10.3389/fmicb.2023.1256878. eCollection 2023. Front Microbiol. 2023. PMID: 37744925 Free PMC article. Review.
-
Postbiotics: emerging therapeutic approach in diabetic retinopathy.Front Microbiol. 2024 Mar 4;15:1359949. doi: 10.3389/fmicb.2024.1359949. eCollection 2024. Front Microbiol. 2024. PMID: 38500583 Free PMC article. Review.
-
GSH-Independent Induction of ER Stress during Hypoglycaemia in the Retinal Cells of Mice.J Clin Med. 2021 Jun 7;10(11):2529. doi: 10.3390/jcm10112529. J Clin Med. 2021. PMID: 34200353 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

