Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias
- PMID: 28128201
- PMCID: PMC5290156
- DOI: 10.1038/ncomms14155
Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias
Abstract
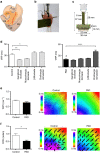
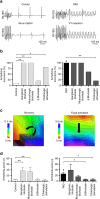
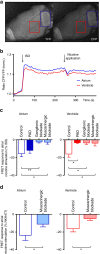
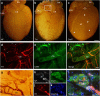
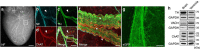
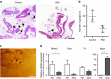
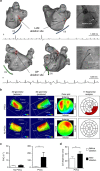
The parasympathetic nervous system plays an important role in the pathophysiology of atrial fibrillation. Catheter ablation, a minimally invasive procedure deactivating abnormal firing cardiac tissue, is increasingly becoming the therapy of choice for atrial fibrillation. This is inevitably associated with the obliteration of cardiac cholinergic neurons. However, the impact on ventricular electrophysiology is unclear. Here we show that cardiac cholinergic neurons modulate ventricular electrophysiology. Mechanical disruption or pharmacological blockade of parasympathetic innervation shortens ventricular refractory periods, increases the incidence of ventricular arrhythmia and decreases ventricular cAMP levels in murine hearts. Immunohistochemistry confirmed ventricular cholinergic innervation, revealing parasympathetic fibres running from the atria to the ventricles parallel to sympathetic fibres. In humans, catheter ablation of atrial fibrillation, which is accompanied by accidental parasympathetic and concomitant sympathetic denervation, raises the burden of premature ventricular complexes. In summary, our results demonstrate an influence of cardiac cholinergic neurons on the regulation of ventricular function and arrhythmogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Savage N. Physiology: beating stroke. Nature 493, S12–S13 (2013). - PubMed
-
- Pappone C. et al.. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 109, 327–334 (2004). - PubMed
-
- Katritsis D. G. et al.. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J. Am. Coll. Cardiol. 62, 2318–2325 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

