A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1α stability
- PMID: 28128204
- PMCID: PMC5290148
- DOI: 10.1038/ncomms14186
A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1α stability
Abstract
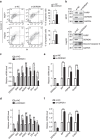
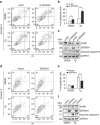
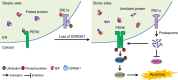
Disturbance of endoplasmic reticulum (ER) homoeostasis induces ER stress and leads to activation of the unfolded protein response (UPR), which is an adaptive reaction that promotes cell survival or triggers apoptosis, when homoeostasis is not restored. DDRGK1 is an ER membrane protein and a critical component of the ubiquitin-fold modifier 1 (Ufm1) system. However, the functions and mechanisms of DDRGK1 in ER homoeostasis are largely unknown. Here, we show that depletion of DDRGK1 induces ER stress and enhances ER stress-induced apoptosis in both cancer cells and hematopoietic stem cells (HSCs). Depletion of DDRGK1 represses IRE1α-XBP1 signalling and activates the PERK-eIF2α-CHOP apoptotic pathway by targeting the ER-stress sensor IRE1α. We further demonstrate that DDRGK1 regulates IRE1α protein stability via its interaction with the kinase domain of IRE1α, which is dependent on its ufmylation modification. Altogether, our results provide evidence that DDRGK1 is essential for ER homoeostasis regulation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
Loss of DDRGK1 impairs IRE1α UFMylation in spondyloepiphyseal dysplasia.Int J Biol Sci. 2023 Sep 4;19(15):4709-4725. doi: 10.7150/ijbs.82765. eCollection 2023. Int J Biol Sci. 2023. PMID: 37781516 Free PMC article.
-
ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras.Proc Natl Acad Sci U S A. 2017 Sep 12;114(37):9900-9905. doi: 10.1073/pnas.1701757114. Epub 2017 Aug 28. Proc Natl Acad Sci U S A. 2017. PMID: 28847931 Free PMC article.
-
Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease.J Biol Chem. 2019 Dec 6;294(49):18726-18741. doi: 10.1074/jbc.REV119.007036. Epub 2019 Oct 30. J Biol Chem. 2019. PMID: 31666338 Free PMC article. Review.
-
Molecular Pathways: Immunosuppressive Roles of IRE1α-XBP1 Signaling in Dendritic Cells of the Tumor Microenvironment.Clin Cancer Res. 2016 May 1;22(9):2121-6. doi: 10.1158/1078-0432.CCR-15-1570. Epub 2016 Mar 15. Clin Cancer Res. 2016. PMID: 26979393 Free PMC article. Review.
Cited by
-
Early Emergence of Adaptive Mechanisms Sustaining Ig Production: Application to Antibody Therapy.Front Immunol. 2021 Apr 29;12:671998. doi: 10.3389/fimmu.2021.671998. eCollection 2021. Front Immunol. 2021. PMID: 33995412 Free PMC article. Review.
-
Generation of the UFM1 Toolkit for Profiling UFM1-Specific Proteases and Ligases.Angew Chem Int Ed Engl. 2018 Oct 22;57(43):14164-14168. doi: 10.1002/anie.201809232. Epub 2018 Oct 1. Angew Chem Int Ed Engl. 2018. PMID: 30188611 Free PMC article.
-
cGAS in nucleus: The link between immune response and DNA damage repair.Front Immunol. 2022 Dec 15;13:1076784. doi: 10.3389/fimmu.2022.1076784. eCollection 2022. Front Immunol. 2022. PMID: 36591232 Free PMC article.
-
The UFMylation pathway is impaired in Alzheimer's disease.Mol Neurodegener. 2024 Dec 18;19(1):97. doi: 10.1186/s13024-024-00784-y. Mol Neurodegener. 2024. PMID: 39696466 Free PMC article.
-
Endoplasmic Reticulum Stress in Acute Myeloid Leukemia: Pathogenesis, Prognostic Implications, and Therapeutic Strategies.Int J Mol Sci. 2025 Mar 27;26(7):3092. doi: 10.3390/ijms26073092. Int J Mol Sci. 2025. PMID: 40243748 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

