Proton pumping accompanies calcification in foraminifera
- PMID: 28128216
- PMCID: PMC5290161
- DOI: 10.1038/ncomms14145
Proton pumping accompanies calcification in foraminifera
Abstract
Ongoing ocean acidification is widely reported to reduce the ability of calcifying marine organisms to produce their shells and skeletons. Whereas increased dissolution due to acidification is a largely inorganic process, strong organismal control over biomineralization influences calcification and hence complicates predicting the response of marine calcifyers. Here we show that calcification is driven by rapid transformation of bicarbonate into carbonate inside the cytoplasm, achieved by active outward proton pumping. Moreover, this proton flux is maintained over a wide range of pCO2 levels. We furthermore show that a V-type H+ ATPase is responsible for the proton flux and thereby calcification. External transformation of bicarbonate into CO2 due to the proton pumping implies that biomineralization does not rely on availability of carbonate ions, but total dissolved CO2 may not reduce calcification, thereby potentially maintaining the current global marine carbonate production.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
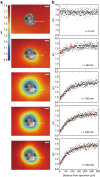
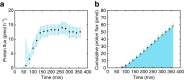
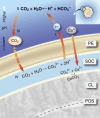
Similar articles
-
Sensitivity of planktic foraminiferal test bulk density to ocean acidification.Sci Rep. 2019 Jul 5;9(1):9803. doi: 10.1038/s41598-019-46041-x. Sci Rep. 2019. PMID: 31278289 Free PMC article.
-
Impact of dissolved CO2 on calcification in two large, benthic foraminiferal species.PLoS One. 2023 Aug 16;18(8):e0289122. doi: 10.1371/journal.pone.0289122. eCollection 2023. PLoS One. 2023. PMID: 37585361 Free PMC article.
-
Deciphering carbon sources of mussel shell carbonate under experimental ocean acidification and warming.Mar Environ Res. 2018 Nov;142:141-146. doi: 10.1016/j.marenvres.2018.10.007. Epub 2018 Oct 13. Mar Environ Res. 2018. PMID: 30337051
-
Ocean acidification: the other CO2 problem.Ann Rev Mar Sci. 2009;1:169-92. doi: 10.1146/annurev.marine.010908.163834. Ann Rev Mar Sci. 2009. PMID: 21141034 Review.
-
Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification.Ann Rev Mar Sci. 2013;5:321-48. doi: 10.1146/annurev-marine-121211-172241. Epub 2012 Jul 9. Ann Rev Mar Sci. 2013. PMID: 22881351 Review.
Cited by
-
Form and function of F-actin during biomineralization revealed from live experiments on foraminifera.Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4111-4116. doi: 10.1073/pnas.1810394116. Epub 2019 Feb 19. Proc Natl Acad Sci U S A. 2019. PMID: 30782789 Free PMC article.
-
Sensitivity of planktic foraminiferal test bulk density to ocean acidification.Sci Rep. 2019 Jul 5;9(1):9803. doi: 10.1038/s41598-019-46041-x. Sci Rep. 2019. PMID: 31278289 Free PMC article.
-
Impact of dissolved CO2 on calcification in two large, benthic foraminiferal species.PLoS One. 2023 Aug 16;18(8):e0289122. doi: 10.1371/journal.pone.0289122. eCollection 2023. PLoS One. 2023. PMID: 37585361 Free PMC article.
-
Phycosphere pH of unicellular nano- and micro- phytoplankton cells and consequences for iron speciation.ISME J. 2022 Oct;16(10):2329-2336. doi: 10.1038/s41396-022-01280-1. Epub 2022 Jul 7. ISME J. 2022. PMID: 35798938 Free PMC article.
-
Mg-rich amorphous to Mg-low crystalline CaCO3 pathway in foraminifera.Heliyon. 2023 Jul 17;9(7):e18331. doi: 10.1016/j.heliyon.2023.e18331. eCollection 2023 Jul. Heliyon. 2023. PMID: 37519760 Free PMC article.
References
-
- Feely R. A. et al.. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004). - PubMed
-
- Sarmiento J. L. & Gruber N. Ocean Biogeochemical Dynamics 528Princeton Univ. Press (2006).
-
- Doney S. C., Fabry V. J., Feely R. A. & Kleypas J. A. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192 (2009). - PubMed
-
- Langer M. R. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J. Eukaryotic Microbiol. 55, 163–169 (2008). - PubMed
-
- Schiebel R. Planktic foraminiferal sedimentation and the marine calcite budget. Global Biogeochem. Cycles 16, 3-1–3-21 (2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

