Pyrintegrin Induces Soft Tissue Formation by Transplanted or Endogenous Cells
- PMID: 28128224
- PMCID: PMC5269584
- DOI: 10.1038/srep36402
Pyrintegrin Induces Soft Tissue Formation by Transplanted or Endogenous Cells
Abstract
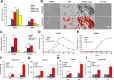
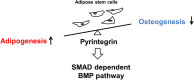
Focal adipose deficiency, such as lipoatrophy, lumpectomy or facial trauma, is a formidable challenge in reconstructive medicine, and yet scarcely investigated in experimental studies. Here, we report that Pyrintegrin (Ptn), a 2,4-disubstituted pyrimidine known to promote embryonic stem cells survival, is robustly adipogenic and induces postnatal adipose tissue formation in vivo of transplanted adipose stem/progenitor cells (ASCs) and recruited endogenous cells. In vitro, Ptn stimulated human adipose tissue derived ASCs to differentiate into lipid-laden adipocytes by upregulating peroxisome proliferator-activated receptor (PPARγ) and CCAAT/enhancer-binding protein-α (C/EBPα), with differentiated cells increasingly secreting adiponectin, leptin, glycerol and total triglycerides. Ptn-primed human ASCs seeded in 3D-bioprinted biomaterial scaffolds yielded newly formed adipose tissue that expressed human PPARγ, when transplanted into the dorsum of athymic mice. Remarkably, Ptn-adsorbed 3D scaffolds implanted in the inguinal fat pad had enhanced adipose tissue formation, suggesting Ptn's ability to induce in situ adipogenesis of endogenous cells. Ptn promoted adipogenesis by upregulating PPARγ and C/EBPα not only in adipogenesis induction medium, but also in chemically defined medium specifically for osteogenesis, and concurrently attenuated Runx2 and Osx via BMP-mediated SMAD1/5 phosphorylation. These findings suggest Ptn's novel role as an adipogenesis inducer with a therapeutic potential in soft tissue reconstruction and augmentation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

