Modeling pre-metastatic lymphvascular niche in the mouse ear sponge assay
- PMID: 28128294
- PMCID: PMC5270255
- DOI: 10.1038/srep41494
Modeling pre-metastatic lymphvascular niche in the mouse ear sponge assay
Abstract
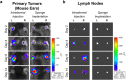
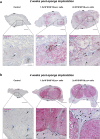
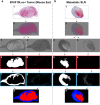
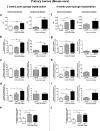
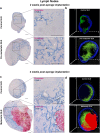
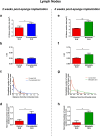
Lymphangiogenesis, the formation of new lymphatic vessels, occurs in primary tumors and in draining lymph nodes leading to pre-metastatic niche formation. Reliable in vivo models are becoming instrumental for investigating alterations occurring in lymph nodes before tumor cell arrival. In this study, we demonstrate that B16F10 melanoma cell encapsulation in a biomaterial, and implantation in the mouse ear, prevents their rapid lymphatic spread observed when cells are directly injected in the ear. Vascular remodeling in lymph nodes was detected two weeks after sponge implantation, while their colonization by tumor cells occurred two weeks later. In this model, a huge lymphangiogenic response was induced in primary tumors and in pre-metastatic and metastatic lymph nodes. In control lymph nodes, lymphatic vessels were confined to the cortex. In contrast, an enlargement and expansion of lymphatic vessels towards paracortical and medullar areas occurred in pre-metastatic lymph nodes. We designed an original computerized-assisted quantification method to examine the lymphatic vessel structure and the spatial distribution. This new reliable and accurate model is suitable for in vivo studies of lymphangiogenesis, holds promise for unraveling the mechanisms underlying lymphatic metastases and pre-metastatic niche formation in lymph nodes, and will provide new tools for drug testing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

