RNA helicase DDX3 maintains lipid homeostasis through upregulation of the microsomal triglyceride transfer protein by interacting with HNF4 and SHP
- PMID: 28128295
- PMCID: PMC5269733
- DOI: 10.1038/srep41452
RNA helicase DDX3 maintains lipid homeostasis through upregulation of the microsomal triglyceride transfer protein by interacting with HNF4 and SHP
Abstract
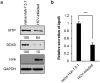
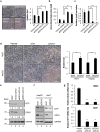
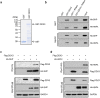
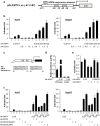
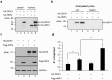
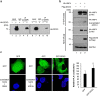
Multifunctional RNA helicase DDX3 participates in HCV infection, one of the major causes of hepatic steatosis. Here, we investigated the role of DDX3 in hepatic lipid metabolism. We found that HCV infection severely reduced DDX3 expression. Analysis of intracellular triglyceride and secreted ApoB indicated that lipid accumulations were increased while ApoB secretion were decreased in DDX3 knockdown HuH7 and HepG2 cell lines. Down-regulation of DDX3 significantly decreased protein and transcript expression of microsomal triglyceride transfer protein (MTP), a key regulator of liver lipid homeostasis. Moreover, DDX3 interacted with hepatocyte nuclear factor 4 (HNF4) and small heterodimer partner (SHP), and synergistically up-regulated HNF4-mediated transactivation of MTP promoter via its ATPase activity. Further investigation revealed that DDX3 interacted with CBP/p300 and increased the promoter binding affinity of HNF4 by enhancing HNF4 acetylation. Additionally, DDX3 partially relieved the SHP-mediated suppression on MTP promoter by competing with SHP for HNF4 binding which disrupted the inactive HNF4/SHP heterodimer while promoted the formation of the active HNF4 homodimer. Collectively, these results imply that DDX3 regulates MTP gene expression and lipid homeostasis through interplay with HNF4 and SHP, which may also reveal a novel mechanism of HCV-induced steatosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
The metabolic regulator small heterodimer partner contributes to the glucose and lipid homeostasis abnormalities induced by hepatitis C virus infection.Metabolism. 2019 Nov;100:153954. doi: 10.1016/j.metabol.2019.153954. Epub 2019 Aug 8. Metabolism. 2019. PMID: 31400386
-
Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4.J Biol Chem. 2004 Oct 29;279(44):45685-92. doi: 10.1074/jbc.M404255200. Epub 2004 Aug 26. J Biol Chem. 2004. PMID: 15337761
-
Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression.Hepatology. 2003 Mar;37(3):622-31. doi: 10.1053/jhep.2003.50100. Hepatology. 2003. PMID: 12601360
-
Structure and function of the atypical orphan nuclear receptor small heterodimer partner.Int Rev Cytol. 2007;261:117-58. doi: 10.1016/S0074-7696(07)61003-1. Int Rev Cytol. 2007. PMID: 17560281 Review.
-
Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism.Curr Opin Lipidol. 2008 Jun;19(3):277-84. doi: 10.1097/MOL.0b013e3282feea85. Curr Opin Lipidol. 2008. PMID: 18460919 Review.
Cited by
-
Non-vesicular Lipid Transport Machinery in Entamoeba histolytica.Front Cell Infect Microbiol. 2018 Sep 19;8:315. doi: 10.3389/fcimb.2018.00315. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30283742 Free PMC article. Review.
-
p300/CBP as a Key Nutritional Sensor for Hepatic Energy Homeostasis and Liver Fibrosis.Biomed Res Int. 2018 May 15;2018:8168791. doi: 10.1155/2018/8168791. eCollection 2018. Biomed Res Int. 2018. PMID: 29862292 Free PMC article. Review.
-
Whole Lotta Lipids-from HCV RNA Replication to the Mature Viral Particle.Int J Mol Sci. 2020 Apr 21;21(8):2888. doi: 10.3390/ijms21082888. Int J Mol Sci. 2020. PMID: 32326151 Free PMC article. Review.
-
A novel reporter for helicase activity in translation uncovers DDX3X interactions.RNA. 2024 Jul 16;30(8):1041-1057. doi: 10.1261/rna.079837.123. RNA. 2024. PMID: 38697667 Free PMC article.
-
Non-Vesicular Lipid Transport Machinery in Leishmania donovani: Functional Implications in Host-Parasite Interaction.Int J Mol Sci. 2023 Jun 26;24(13):10637. doi: 10.3390/ijms241310637. Int J Mol Sci. 2023. PMID: 37445815 Free PMC article. Review.
References
-
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 (2002). - PubMed
-
- Ibrahim M. A., Kelleni M. & Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 92, 114–118 (2013). - PubMed
-
- Wetterau J. R. et al.. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

