Multilevel interaction of the DnaK/DnaJ(HSP70/HSP40) stress-responsive chaperone machine with the central metabolism
- PMID: 28128357
- PMCID: PMC5269706
- DOI: 10.1038/srep41341
Multilevel interaction of the DnaK/DnaJ(HSP70/HSP40) stress-responsive chaperone machine with the central metabolism
Abstract
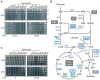
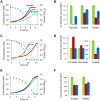
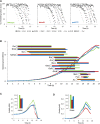
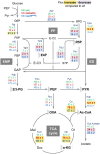
Networks of molecular chaperones maintain cellular protein homeostasis by acting at nearly every step in the biogenesis of proteins and protein complexes. Herein, we demonstrate that the major chaperone DnaK/HSP70 of the model bacterium Escherichia coli is critical for the proper functioning of the central metabolism and for the cellular response to carbon nutrition changes, either directly or indirectly via the control of the heat-shock response. We identified carbon sources whose utilization was positively or negatively affected by DnaK and isolated several central metabolism genes (among other genes identified in this work) that compensate for the lack of DnaK and/or DnaK/Trigger Factor chaperone functions in vivo. Using carbon sources with specific entry points coupled to NMR analyses of real-time carbon assimilation, metabolic coproducts production and flux rearrangements, we demonstrate that DnaK significantly impacts the hierarchical order of carbon sources utilization, the excretion of main coproducts and the distribution of metabolic fluxes, thus revealing a multilevel interaction of DnaK with the central metabolism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Bukau B., Weissman J. & Horwich A. Molecular chaperones and protein quality control. Cell 125, 443–451 (2006). - PubMed
-
- Genevaux P., Georgopoulos C. & Kelley W. L. The Hsp70 chaperone machines of Escherichia coli: a paradigm for the repartition of chaperone functions. Mol. Microbiol. 66, 840–857 (2007). - PubMed
-
- Kramer G., Boehringer D., Ban N. & Bukau B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nature Structural & Molecular Biology 16, 589–597 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

