A tachykinin-like neuroendocrine signalling axis couples central serotonin action and nutrient sensing with peripheral lipid metabolism
- PMID: 28128367
- PMCID: PMC5290170
- DOI: 10.1038/ncomms14237
A tachykinin-like neuroendocrine signalling axis couples central serotonin action and nutrient sensing with peripheral lipid metabolism
Abstract
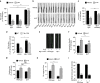
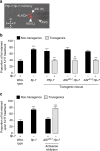
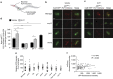
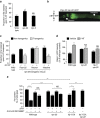
Serotonin, a central neuromodulator with ancient ties to feeding and metabolism, is a major driver of body fat loss. However, mechanisms by which central serotonin action leads to fat loss remain unknown. Here, we report that the FLP-7 neuropeptide and its cognate receptor, NPR-22, function as the ligand-receptor pair that defines the neuroendocrine axis of serotonergic body fat loss in Caenorhabditis elegans. FLP-7 is secreted as a neuroendocrine peptide in proportion to fluctuations in neural serotonin circuit functions, and its release is regulated from secretory neurons via the nutrient sensor AMPK. FLP-7 acts via the NPR-22/Tachykinin2 receptor in the intestine and drives fat loss via the adipocyte triglyceride lipase ATGL-1. Importantly, this ligand-receptor pair does not alter other serotonin-dependent behaviours including food intake. For global modulators such as serotonin, the use of distinct neuroendocrine peptides for each output may be one means to achieve phenotypic selectivity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

