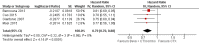Second-line systemic therapy for metastatic colorectal cancer
- PMID: 28128439
- PMCID: PMC6464923
- DOI: 10.1002/14651858.CD006875.pub3
Second-line systemic therapy for metastatic colorectal cancer
Abstract
Background: The therapeutic management of people with metastatic colorectal cancer (CRC) who did not respond to first-line treatment represents a formidable challenge.
Objectives: To determine the efficacy and toxicity of second-line systemic therapy in people with metastatic CRC.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 4), Ovid MEDLINE (1950 to May 2016), Ovid MEDLINE In-process & Other Non-Indexed Citations (1946 to May 2016) and Ovid Embase (1974 to May 2016). There were no language or date of publication restrictions.
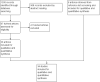
Selection criteria: Randomized controlled trials (RCTs) assessing the efficacy (survival, tumour response) and toxicity (incidence of severe adverse effects (SAEs)) of second-line systemic therapy (single or combined treatment with any anticancer drug, at any dose and number of cycles) in people with metastatic CRC that progressed, recurred or did not respond to first-line systemic therapy.
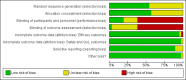
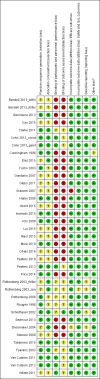
Data collection and analysis: Authors performed a descriptive analysis of each included RCT in terms of primary (survival) and secondary (tumour response, toxicity) endpoints. In the light of the variety of drug regimens tested in the included trials, we could carry out meta-analysis considering classes of (rather than single) anticancer regimens; to this aim, we applied the random-effects model to pool the data. We used hazard ratios (HRs) and risk ratios (RRs) to describe the strength of the association for survival (overall (OS) and progression-free survival (PFS)) and dichotomous (overall response rate (ORR) and SAE rate) data, respectively, with 95% confidence intervals (CI).
Main results: Thirty-four RCTs (enrolling 13,787 participants) fulfilled the eligibility criteria. Available evidence enabled us to address multiple clinical issues regarding the survival effects of second-line systemic therapy of people with metastatic CRC.1. Chemotherapy (irinotecan) was more effective than best supportive care (HR for OS: 0.58, 95% CI 0.43 to 0.80; 1 RCT; moderate-quality evidence); 2. modern chemotherapy (FOLFOX (5-fluorouracil plus leucovorin plus oxaliplatin), irinotecan) is more effective than outdated chemotherapy (5-fluorouracil) (HR for PFS: 0.59, 95% CI 0.49 to 0.73; 2 RCTs; high-quality evidence) (HR for OS: 0.69, 95% CI 0.51 to 0.94; 1 RCT; moderate-quality evidence); 3. irinotecan-based combinations were more effective than irinotecan alone (HR for PFS: 0.68, 95% CI 0.60 to 0.76; 6 RCTs; moderate-quality evidence); 4. targeted agents improved the efficacy of conventional chemotherapy both when considered together (HR for OS: 0.84, 95% CI 0.77 to 0.91; 6 RCTs; high-quality evidence) and when bevacizumab was used alone (HR for PFS: 0.67, 95% CI 0.60 to 0.75; 4 RCTs; high-quality evidence).With regard to secondary endpoints, tumour response rates generally paralleled the survival results; moreover, higher anticancer efficacy was generally associated with worse treatment-related toxicity, with the important exception of bevacizumab-containing regimens, where the addition of the targeted agent to chemotherapy did not result in a significant increase in the rate of SAE. Finally, we found that oral (instead of intravenous) fluoropyrimidines significantly reduced the incidence of adverse effects (without compromising efficacy) in people treated with oxaliplatin-based regimens.We could not draw any conclusions on other debated aspects in this field of oncology, such as ranking of treatments (not all possible comparisons have been tested and many comparisons were based on single trials enrolling a small number of participants) and quality of life (virtually no data available).
Authors' conclusions: Systemic therapy offers a survival benefit to people with metastatic CRC who did not respond to first-line treatment, especially when targeted agents are combined with conventional chemotherapeutic drugs. Further research is needed to define the optimal regimen and to identify people who most benefit from each treatment.
Conflict of interest statement
None of the authors have any known conflicts of interest to declare.
Update of
-
Second-line chemotherapy in advanced and metastatic CRC.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD006875. doi: 10.1002/14651858.CD006875.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2017 Jan 27;1:CD006875. doi: 10.1002/14651858.CD006875.pub3. PMID: 19370656 Updated.
Similar articles
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
Oral versus intravenous fluoropyrimidines for colorectal cancer.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD008398. doi: 10.1002/14651858.CD008398.pub2. Cochrane Database Syst Rev. 2017. PMID: 28752564 Free PMC article.
-
Transarterial (chemo)embolisation versus systemic chemotherapy for colorectal cancer liver metastases.Cochrane Database Syst Rev. 2024 Aug 9;8(8):CD012757. doi: 10.1002/14651858.CD012757.pub2. Cochrane Database Syst Rev. 2024. PMID: 39119869 Free PMC article. Review.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD007047. doi: 10.1002/14651858.CD007047.pub2. Cochrane Database Syst Rev. 2017. PMID: 28654140 Free PMC article.
Cited by
-
The paradigm of tumor shrinkage and rapid liver remnant hypertrophy for conversion of initially unresectable colorectal liver metastasis: a case report and literature review.World J Surg Oncol. 2017 Aug 3;15(1):148. doi: 10.1186/s12957-017-1212-6. World J Surg Oncol. 2017. PMID: 28774330 Free PMC article. Review.
-
Biweekly Raltitrexed Combined With Irinotecan as Second-Line Therapy for Patients With Metastatic Colorectal Cancer: A Phase II Trial.Cancer Control. 2022 Jan-Dec;29:10732748221080332. doi: 10.1177/10732748221080332. Cancer Control. 2022. PMID: 35343258 Free PMC article. Clinical Trial.
-
Individualized prediction of survival benefit from primary tumor resection for patients with unresectable metastatic colorectal cancer.World J Surg Oncol. 2020 Aug 3;18(1):193. doi: 10.1186/s12957-020-01972-y. World J Surg Oncol. 2020. PMID: 32746835 Free PMC article.
-
Mitomycin C and capecitabine: An additional option as an advanced line therapy in patients with metastatic colorectal cancer.World J Gastrointest Oncol. 2023 Nov 15;15(11):1913-1924. doi: 10.4251/wjgo.v15.i11.1913. World J Gastrointest Oncol. 2023. PMID: 38077638 Free PMC article.
-
USP11 induce resistance to 5-Fluorouracil in Colorectal Cancer through activating autophagy by stabilizing VCP.J Cancer. 2021 Feb 22;12(8):2317-2325. doi: 10.7150/jca.52158. eCollection 2021. J Cancer. 2021. PMID: 33758608 Free PMC article.
References
References to studies included in this review
Bendell 2013_folfiri {published data only}
-
- Bendell JC, Tournigand C, Swieboda‐Sadlej A, Barone C, Wainberg ZA, Kim JG, et al. Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX‐6 after failure of first‐line therapy for metastatic colorectal cancer: a randomized phase II study. Clinical Colorectal Cancer 2013;12:239‐47. - PubMed
Bendell 2013_folfox {published data only}
-
- Bendell JC, Tournigand C, Swieboda‐Sadlej A, Barone C, Wainberg ZA, Kim JG, et al. Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX‐6 after failure of first‐line therapy for metastatic colorectal cancer: a randomized phase II study. Clinical Colorectal Cancer 2013;12:239‐47. - PubMed
Bennouna 2013 {published data only}
-
- Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncology 2013;14:29‐37. - PubMed
Cao 2015 {published data only}
-
- Cao R, Zhang S, Ma D, Hu L. A multicenter randomized phase II clinical study of bevacizumab plus irinotecan, 5‐fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second‐line treatment for Chinese patients with metastatic colorectal cancer. Medical Oncology 2015;32:325. - PubMed
Clarke 2011 {published data only}
-
- Clarke SJ, Yip S, Brown C, Hazel GA, Ransom DT, Goldstein D, et al on behalf of the Australasian Gastro‐Intestinal Trials Group. Single‐agent irinotecan or 5‐fluorouracil and leucovorin (FOLFIRI) as second‐line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta‐analysis. European Journal of Cancer 2011;47(12):1826‐36. - PubMed
Cohn 2013_conat {published data only}
-
- Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, et al. A randomized, placebo‐controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second‐line treatment of mutant KRAS metastatic colorectal cancer. Annals of Oncology 2013;24:1777‐85. - PubMed
Cohn 2013_ganit {published data only}
-
- Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, et al. A randomized, placebo‐controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second‐line treatment of mutant KRAS metastatic colorectal cancer. Annals of Oncology 2013;24:1777‐85. - PubMed
Cunningham 1998 {published data only}
-
- Cunningham D, Pyrhönen S, James RD, Punt CJA, Hickish TF, Heikkila R, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413‐8. - PubMed
Élez 2015 {published data only}
-
- Élez E, Kocáková I, Höhler T, Martens UM, Bokemeyer C, Cutsem E, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild‐type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Annals of Oncology 2015;26:132‐40. - PubMed
Fuchs 2003 {published data only}
-
- Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. Phase III comparison of two irinotecan dosing regimens in second‐line therapy of metastatic colorectal cancer. Journal of Clinical Oncology 2003;21(5):807‐14. - PubMed
Giantonio 2007 {published data only}
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 2007;25:1539‐44. - PubMed
Gibbs 2011 {published data only}
-
- Gibbs P, Clingan PR, Ganju V, Strickland AH, Wong SS, Tebbutt NC, et al. Hyaluronan‐irinotecan improves progression‐free survival in 5‐fluorouracil refractory patients with metastatic colorectal cancer: a randomized phase II trial. Cancer Chemotherapy and Pharmacology 2011;67(1):153‐63. - PubMed
Graeven 2007 {published data only}
-
- Graeven U, Arnold D, Reinacher‐Schick A, Heuer T, Nusch A, Porschen R, et al. A randomised phase II study of irinotecan in combination with 5‐FU/FA compared with irinotecan alone as second‐line treatment of patients with metastatic colorectal carcinoma. Onkologie 2007;30(4):169‐74. - PubMed
Haller 2008 {published data only}
-
- Haller DG, Rothenberg ML, Wong AO, Koralewski PM, Miller WH Jr, Bodoky G, et al. Oxaliplatin plus irinotecan compared with irinotecan alone as second‐line treatment after single‐agent fluoropyrimidine therapy for metastatic colorectal carcinoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2008;26(28):4544‐50. - PubMed
Hecht 2015 {published data only}
-
- Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline‐Burkhardt M, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second‐line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clinical Colorectal Cancer 2015;14:72‐80. - PubMed
Iwamoto 2015 {published data only}
-
- Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, et al. FOLFIRI plus bevacizumab as second‐line therapy in patients with metastatic colorectal cancer after first‐line bevacizumab plus oxaliplatin‐based therapy: the randomized phase III EAGLE study. Annals of Oncology 2015;26:1427‐33. - PMC - PubMed
Kim 2009 {published data only}
-
- Kim GP, Sargent DJ, Mahoney MR, Rowland KM Jr, Philip PA, Mitchell E, et al. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. Journal of Clinical Oncology 2009;27(17):2848‐54. - PMC - PubMed
Liu 2015 {published data only}
-
- Liu Y, Luan L, Wang X. A randomized phase II clinical study of combining panitumumab and bevacizumab, plus irinotecan, 5‐fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second‐line treatment for patients with metastatic colorectal cancer and KRAS mutation. Journal of OncoTargets and Therapy 2015;8:1061‐8. - PMC - PubMed
Masi 2015 {published data only}
-
- Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, et al. BEBYP Study Investigators. Continuation or reintroduction of bevacizumab beyond progression to first‐line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Annals of Oncology 2015;26:724‐30. - PubMed
Muro 2010 {published data only}
-
- Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. Irinotecan plus S‐1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second‐line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non‐inferiority study (FIRIS study). Lancet Oncology 2010;11(9):853‐60. - PubMed
O'Neil 2014 {published data only}
-
- O'Neil BH, Cainap C, Cutsem E, Gorbunova V, Karapetis CS, Berlin J, et al. Randomized phase II open‐label study of mFOLFOX6 in combination with linifanib or bevacizumab for metastatic colorectal cancer. Clinical Colorectal Cancer 2014;13:156‐63. - PubMed
Peeters 2010 {published data only}
-
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second‐line treatment in patients with metastatic colorectal cancer. Journal of Clinical Oncology 2010;28:4706‐13. - PubMed
Peeters 2013 {published data only}
-
- Peeters M, Strickland AH, Lichinitser M, Suresh AV, Manikhas G, Shapiro J, et al. A randomised, double‐blind, placebo‐controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. British Journal of Cancer 2013;108:503‐11. - PMC - PubMed
Price 2014 {published data only}
-
- Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy‐refractory wild‐type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open‐label, non‐inferiority phase 3 study. Lancet Oncology 2014;15:569‐79. - PubMed
Rothenberg 2003_folfox {published data only}
-
- Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluorouracil‐leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil‐leucovorin: interim results of a phase III trial. Journal of Clinical Oncology 2003;21(11):2059‐69. - PubMed
Rothenberg 2003_oxa {published data only}
-
- Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluorouracil‐leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil‐leucovorin: interim results of a phase III trial. Journal of Clinical Oncology 2003;21(11):2059‐69. - PubMed
Rothenberg 2008 {published data only}
-
- Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, et al. Capecitabine plus oxaliplatin (XELOX) versus 5‐fluorouracil/folinic acid plus oxaliplatin (FOLFOX‐4) as second‐line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Annals of Oncology 2008;19:1720‐6. - PubMed
Rougier 1998 {published data only}
-
- Rougier P, Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998;352(9138):1407‐12. - PubMed
Scheithauer 2002 {published data only}
-
- Scheithauer W, Kornek GV, Brugger S, Ullrich‐Pur H, Valencak J, Raderer M, et al. Randomized phase II study of irinotecan plus mitomycin C vs. oxaliplatin plus mitomycin C in patients with advanced fluoropyrimidine/leucovorin‐pretreated colorectal cancer. Cancer Investigation 2002;20(1):60‐8. - PubMed
Seymour 2013 {published data only}
-
- Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild‐type, fluorouracil‐resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncology 2013;14:749‐59. - PMC - PubMed
Shoemaker 2004 {published data only}
Sobrero 2008 {published data only}
-
- Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. Journal of Clinical Oncology 2008;26:2311‐9. - PubMed
Tabernero 2015 {published data only}
-
- Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia‐Carbonero R, et al. RAISE Study Investigators. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double‐blind, multicentre, phase 3 study. Lancet Oncology 2015;16:499‐508. - PubMed
Tsavaris 2003 {published data only}
-
- Tsavaris N, Ziras N, Kosmas C, Giannakakis T, Gouveris P, Vadiaka M. Two different schedules of irinotecan (CPT‐11) in patients with advanced colorectal carcinoma relapsing after a 5‐fluorouracil and leucovorin combination: a randomised study. Cancer Chemotherapy and Pharmacology 2003;52(6):514‐9. - PubMed
Van Cutsem 2011 {published data only}
-
- Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, et al. Randomized, placebo‐controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. Journal of Clinical Oncology 2011;29:2004‐10. - PubMed
Van Cutsem 2012 {published data only}
-
- Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. Journal of Clinical Oncology 2012;30:3499‐506. - PubMed
Viéitez 2011 {published data only}
-
- Viéitez JM, Valladares M, Peláez I, Sande González L, García‐Foncillas J, García‐López JL, et al. A randomized phase II study of raltitrexed and gefitinib versus raltitrexed alone as second line chemotherapy in patients with colorectal cancer (1839IL/0143). Investigational New Drugs 2011;29:1038‐44. - PubMed
References to studies excluded from this review
Amado 2008 {published data only}
-
- Amado RG, Wolf M, Peeters M, Cutsem E, Siena S, Freeman DJ, et al. Wild‐type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of Clinical Oncology 2008;26:1626‐34. - PubMed
Aranda 1998 {published data only}
-
- Aranda E, Díaz‐Rubio E, Cervantes A, Antón‐Torres A, Carrato A, Massutí T, et al. Randomized trial comparing monthly low‐dose leucovorin and fluorouracil bolus with weekly high‐dose 48‐hour continuous‐infusion fluorouracil for advanced colorectal cancer: a Spanish Cooperative Group for Gastrointestinal Tumor Therapy (TTD) study. Annals of Oncology 1998;9:727‐31. - PubMed
Chau 2004 {published data only}
-
- Chau I, Norman AR, Cunningham D, Waters JS, Topham C, Middleton G, et al. Elderly patients with fluoropyrimidine and thymidylate synthase inhibitor‐resistant advanced colorectal cancer derive similar benefit without excessive toxicity when treated with irinotecan monotherapy. British Journal of Cancer 2004;91(8):1453‐8. - PMC - PubMed
Chibaudel 2016 {published data only}
-
- Chibaudel B, Maindrault‐Gœbel F, Bachet JB, Louvet C, Khalil A, Dupuis O, et al. PEPCOL: a GERCOR randomized phase II study of nanoliposomal irinotecan PEP02 (MM‐398) or irinotecan with leucovorin/5‐fluorouracil as second‐line therapy in metastatic colorectal cancer. Cancer Medicine 2016;5:676‐83. - PMC - PubMed
Comella 2000 {published data only}
-
- Comella P, Vita F, Mancarella S, Lucia L, Biglietto M, Casaretti R, et al. Biweekly irinotecan or raltitrexed plus 6S‐leucovorin and bolus 5‐fluorouracil in advanced colorectal carcinoma: a Southern Italy Cooperative Oncology Group phase II‐III randomized trial. Annals of Oncology 2000;11(10):1323‐33. - PubMed
Comella 2002 {published data only}
-
- Comella P, Crucitta E, Vita F, Lucia L, Farris A, Gaizo F, et al. Addition of either irinotecan or methotrexate to bolus 5‐fluorouracil and high‐dose folinic acid every 2 weeks in advanced colorectal carcinoma: a randomised study by the Southern Italy Cooperative Oncology Group. Annals of Oncology 2002;13(6):866‐73. - PubMed
Cunningham 2004 {published data only}
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. New England Journal of Medicine 2004;351:337‐45. - PubMed
Ducreux 2011 {published data only}
-
- Ducreux M, Malka D, Mendiboure J, Etienne PL, Texereau P, Auby D, et al. Fédération Francophone de Cancérologie Digestive (FFCD) 2000‐05 Collaborative Group. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000‐05): an open‐label, randomised, phase 3 trial. Lancet Oncology 2011;12:1032‐44. - PubMed
Garrett 2013 {published data only}
-
- Garrett CR, Bekaii‐Saab TS, Ryan T, Fisher GA, Clive S, Kavan P, et al. Randomized phase 2 study of pegylated SN‐38 (EZN‐2208) or irinotecan plus cetuximab in patients with advanced colorectal cancer. Cancer 2013;119:4223‐30. - PubMed
Grothey 2013 {published data only}
-
- Grothey A, Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet Oncology 2013;381:303‐12. - PubMed
Hendlisz 2010 {published data only}
-
- Hendlisz A, Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium‐90 resin microspheres radioembolization for liver‐limited metastatic colorectal cancer refractory to standard chemotherapy. Journal of Clinical Oncology 2010;28:3687‐94. - PubMed
Hurwitz 2004 {published data only}
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 2004;350:2335‐42. - PubMed
Jonker 2007 {published data only}
-
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. New England Journal of Medicine 2007;357:2040‐8. - PubMed
Kabbinavar 2003 {published data only}
-
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer . Journal of Clinical Oncology 2003;21:60‐5. - PubMed
Kemeny 1993 {published data only}
-
- Kemeny N, Cohen A, Seiter K, Conti JA, Sigurdson ER, Tao Y, et al. Randomized trial of hepatic arterial floxuridine, mitomycin, and carmustine versus floxuridine alone in previously treated patients with liver metastases from colorectal cancer. Journal of Clinical Oncology 1993;11(2):330‐5. - PubMed
Koopman 2007 {published data only}
-
- Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135‐42. - PubMed
Labianca 2011 {published data only}
-
- Labianca R, Sobrero A, Isa L, Cortesi E, Barni S, Nicolella D, et al. Italian Group for the Study of Gastrointestinal Cancer‐GISCAD. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised 'GISCAD' trial. Annals of Oncology 2011;22:1236‐42. - PubMed
Lacouture 2010 {published data only}
-
- Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open‐label, randomized trial evaluating the impact of a pre‐emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. Journal of Clinical Oncology 2010;28:1351‐7. - PubMed
Lal 2004 {published data only}
-
- Lal R, Dickson J, Cunningham D, Chau I, Norman AR, Ross PJ, et al. A randomised trial comparing defined‐duration with continuous irinotecan until disease progression in fluoropyrimidine and thymidylate synthase inhibitor‐resistant advanced colorectal cancer. Journal of Clinical Oncology 2004;22(15):3023‐31. - PubMed
Levi 1997 {published data only}
-
- Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997;350:681‐6. - PubMed
Lin 2014 {published data only}
Mandalá 2009 {published data only}
-
- Labianca R, Floriani I, Cortesi L, Zaniboni A, Marangolo M, et al. Alternating versus continuous "FOLFIRI" in advanced colorectal cancer (ACC): a randomized "GISCAD" trial. Journal of Clinical Oncology 2006;24 (Suppl):3505.
-
- Mandalá M, Barni S, Floriani I, Isa L, Fornarini G, Marangolo M, et al. Incidence and clinical implications of venous thromboembolism in advanced colorectal cancer patients: the 'GISCAD‐alternating schedule' study findings. European Journal of Cancer 2009;45(1):65‐73. - PubMed
Maughan 2003 {published data only}
-
- Maughan TS, James RD, Kerr DJ, Ledermann JA, Seymour MT, Topham C, et al. Medical Research Council Colorectal Cancer Group. Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: a multicentre randomised trial. Lancet 2003;361:457‐64. - PubMed
Mayer 2015 {published data only}
-
- Mayer RJ, Cutsem E, Falcone A, Yoshino T, Garcia‐Carbonero R, Mizunuma N, et al. RECOURSE Study Group. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. New England Journal of Medicine 2015;372:1909‐19. - PubMed
Muro 2016 {published data only}
-
- Muro K, Kim T, Park Y, Uetake H, Nishina T, Nozawa H, et al. A multinational, randomized, phase III trial of XELIRI (+bevacizumab) versus FOLFIRI (+bevacizumab) as the second‐line chemotherapy for metastatic colorectal cancer: Asian XELIRI project (AXEPT). Journal of Clinical Oncology 2016;Suppl 4S:TPS780. - PMC - PubMed
Rao 2004 {published data only}
-
- Rao S, Cunningham D, Gramont A, Scheithauer W, Smakal M, Humblet Y, et al. Phase III double‐blind placebo‐controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. Journal of Clinical Oncology 2004;22(19):3950‐7. - PubMed
Reidy 2010 {published data only}
-
- Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman‐Davis N, et al. Randomized, phase II study of the insulin‐like growth factor‐1 receptor inhibitor IMC‐A12, with or without cetuximab, in patients with cetuximab‐ or panitumumab‐refractory metastatic colorectal cancer. Journal of Clinical Oncology. 2010;28:4240‐6. - PMC - PubMed
Rougier 2002 {published data only}
-
- Rougier P, Lepille D, Bennouna J, Marre A, Ducreux M, Mignot L, et al. Antitumour activity of three second‐line treatment combinations in patients with metastatic colorectal cancer after optimal 5‐FU regimen failure: a randomised, multicentre phase II study. Annals of Oncology 2002;13:1558‐67. - PubMed
Saltz 2000 {published data only}
-
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. New England Journal of Medicine 2000;343:905‐14. - PubMed
Saltz 2007 {published data only}
-
- Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan‐refractory colorectal cancer: the BOND‐2 study. Journal of Clinical Oncology 2007;25:4557‐61. - PubMed
Seymour 2007 {published data only}
-
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al for the FOCUS Trial Investigators and the National Cancer Research Institute Colorectal Clinical Studies Group. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143‐52. - PubMed
Siu 2013 {published data only}
-
- Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, et al. Phase III randomized, placebo‐controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy‐refractory, wild‐type K‐RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. Journal of Clinical Oncology 2013;31:2477‐84. - PubMed
Tournigand 2004 {published data only}
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery‐Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. Journal of Clinical Oncology 2004;22:229‐37. - PubMed
Tournigand 2006 {published data only}
-
- Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop‐and‐Go fashion in advanced colorectal cancer‐‐a GERCOR study. Journal of Clinical Oncology 2006;24:394‐400. - PubMed
Tsavaris 2007 {published data only}
-
- Tsavaris N, Kosmas C, Skopelitis H, Papadoniou N, Polyzos A, Zografos G, et al. Sequential administration of 5‐fluorouracil (5FU)/leucovorin (LV) followed by irinotecan (CPT‐11) at relapse versus CPT‐11 followed by 5‐FU/LV in advanced colorectal carcinoma. A phase III randomized study. Chemotherapy 2007;53(4):282‐91. - PubMed
Van Cutsem 2005 {published data only}
-
- Cutsem E, Dirix L, Laethem JL, Belle S, Borner M, Gonzalez Baron M, et al. Optimisation of irinotecan dose in the treatment of patients with metastatic colorectal cancer after 5‐FU failure: results from a multinational, randomised phase II study. British Journal of Cancer 2005;92:1055‐62. - PMC - PubMed
Van Cutsem 2007 {published data only}
-
- Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. Journal of Clinical Oncology 2007;25:1658‐64. - PubMed
Van Cutsem 2012_bis {published data only}
-
- Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. Journal of Clinical Oncology 2012;30(23):2861‐8. - PubMed
Van Cutsem 2014 {published data only}
Yasui 2015 {published data only}
-
- Yasui H, Muro K, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. A phase 3 non‐inferiority study of 5‐FU/l‐leucovorin/irinotecan (FOLFIRI) versus irinotecan/S‐1 (IRIS) as second‐line chemotherapy for metastatic colorectal cancer: updated results of the FIRIS study. Journal of Cancer Research in Clinical Oncology 2015;141:153‐60. - PMC - PubMed
Yoshino 2012 {published data only}
-
- Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS‐102 monotherapy for pretreated metastatic colorectal cancer: a double‐blind, randomised, placebo‐controlled phase 2 trial. Lancet Oncology 2012;13:993‐1001. - PubMed
Additional references
Adam 2012
Altman 1999
Arnold 2013
-
- Arnold D, Stein A. New developments in the second‐line treatment of metastatic colorectal cancer: potential place in therapy. Drugs 2013;73:883‐91. - PubMed
Beretta 2013
-
- Beretta GD, Petrelli F, Stinco S, Cabiddu M, Ghilardi M, Squadroni M, et al. FOLFIRI + bevacizumab as second‐line therapy for metastatic colorectal cancer pretreated with oxaliplatin: a pooled analysis of published trials. Medical Oncology 2013;30(1):486. - PubMed
Best 2000
Buyse 2007
-
- Buyse M, Burzykowski T, Carroll K, Michiels S, Sargent DJ, Miller LL, et al. Progression‐free survival is a surrogate for survival in advanced colorectal cancer. Journal of Clinical Oncology 2007;25:5218‐24. - PubMed
CTCAE
-
- Common Terminology Criteria for Adverse Events v3.0 (CTCAE). ctep.cancer.gov/reporting/ctc.html (accessed 16 October 2016).
Cunningham 2010
-
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010;375:1030‐47. - PubMed
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136:E359‐86. - PubMed
Giessen 2015
-
- Giessen C, Laubender RP, Ankerst DP, Stintzing S, Modest DP, Schulz C, et al. Surrogate endpoints in second‐line treatment for mCRC: a systematic literature‐based analysis from 23 randomised trials. Acta Oncologica 2015;54:187‐93. - PubMed
Goldberg 2006
-
- Goldberg M. Therapy for metastatic colorectal cancer. Oncologist 2006;11:981‐7. - PubMed
Grothey 2006
-
- Grothey A. Is there a third‐line therapy for metastatic colorectal cancer?. Seminars in Oncology 2006;33(6 Suppl 11):S36‐8. - PubMed
Gustavsson 2015
-
- Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clinical Colorectal Cancer 2015;14:1‐10. - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380‐2. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kirstein 2014
Marques 2014
NICE 2005
-
- National Institute for Health and Care Excellence. Colorectal cancer (advanced) ‐ irinotecan, oxaliplatin and raltitrexed (review). www.nice.org.uk/TA093.pdf.
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rougier 2005
-
- Rougier P, Lepere C. Second‐line treatment of patients with metastatic colorectal cancer. Seminars in Oncology 2005;32(6 Suppl 9):S48‐54. - PubMed
Saunders 2006
Schmoll 2012
-
- Schmoll HJ, Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Annals Oncology 2012;23:2479‐516. - PubMed
Sterne 2000
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53:1119‐29. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Torre 2015
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65:87‐108. - PubMed
WHO 1979
-
- World Health Organization. Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization, 1979:15‐22.
WHO 2003
-
- World Health Organization/International Agency for Research on Cancer. The World Cancer Report ‐ the major findings. Central European Journal of Public Health 2003;11:177‐9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical