Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support
- PMID: 28128441
- PMCID: PMC5298168
- DOI: 10.1002/14651858.CD011305.pub2
Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support
Update in
-
Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support.Cochrane Database Syst Rev. 2024 May 23;5(5):CD011305. doi: 10.1002/14651858.CD011305.pub3. Cochrane Database Syst Rev. 2024. PMID: 38780066 Free PMC article.
Abstract
Background: Many people diagnosed with haematological malignancies experience anaemia, and red blood cell (RBC) transfusion plays an essential supportive role in their management. Different strategies have been developed for RBC transfusions. A restrictive transfusion strategy seeks to maintain a lower haemoglobin level (usually between 70 g/L to 90 g/L) with a trigger for transfusion when the haemoglobin drops below 70 g/L), whereas a liberal transfusion strategy aims to maintain a higher haemoglobin (usually between 100 g/L to 120 g/L, with a threshold for transfusion when haemoglobin drops below 100 g/L). In people undergoing surgery or who have been admitted to intensive care a restrictive transfusion strategy has been shown to be safe and in some cases safer than a liberal transfusion strategy. However, it is not known whether it is safe in people with haematological malignancies.
Objectives: To determine the efficacy and safety of restrictive versus liberal RBC transfusion strategies for people diagnosed with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without a haematopoietic stem cell transplant (HSCT).
Search methods: We searched for randomised controlled trials (RCTs) and non-randomised trials (NRS) in MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1982), Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 6), and 10 other databases (including four trial registries) to 15 June 2016. We also searched grey literature and contacted experts in transfusion for additional trials. There was no restriction on language, date or publication status.
Selection criteria: We included RCTs and prospective NRS that evaluated a restrictive compared with a liberal RBC transfusion strategy in children or adults with malignant haematological disorders or undergoing HSCT.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
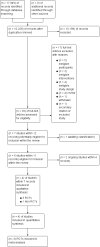
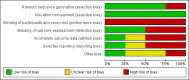
Main results: We identified six studies eligible for inclusion in this review; five RCTs and one NRS. Three completed RCTs (156 participants), one completed NRS (84 participants), and two ongoing RCTs. We identified one additional RCT awaiting classification. The completed studies were conducted between 1997 and 2015 and had a mean follow-up from 31 days to 2 years. One study included children receiving a HSCT (six participants), the other three studies only included adults: 218 participants with acute leukaemia receiving chemotherapy, and 16 with a haematological malignancy receiving a HSCT. The restrictive strategies varied from 70 g/L to 90 g/L. The liberal strategies also varied from 80 g/L to 120 g/L.Based on the GRADE rating methodology the overall quality of the included studies was very low to low across different outcomes. None of the included studies were free from bias for all 'Risk of bias' domains. One of the three RCTs was discontinued early for safety concerns after recruiting only six children, all three participants in the liberal group developed veno-occlusive disease (VOD). Evidence from RCTsA restrictive RBC transfusion policy may make little or no difference to: the number of participants who died within 100 days (two trials, 95 participants (RR: 0.25, 95% CI 0.02 to 2.69, low-quality evidence); the number of participants who experienced any bleeding (two studies, 149 participants; RR:0.93, 95% CI 0.73 to 1.18, low-quality evidence), or clinically significant bleeding (two studies, 149 participants, RR: 1.03, 95% CI 0.75 to 1.43, low-quality evidence); the number of participants who required RBC transfusions (three trials; 155 participants: RR: 0.97, 95% CI 0.90 to 1.05, low-quality evidence); or the length of hospital stay (restrictive median 35.5 days (interquartile range (IQR): 31.2 to 43.8); liberal 36 days (IQR: 29.2 to 44), low-quality evidence).We are uncertain whether the restrictive RBC transfusion strategy: decreases quality of life (one trial, 89 participants, fatigue score: restrictive median 4.8 (IQR 4 to 5.2); liberal median 4.5 (IQR 3.6 to 5) (very low-quality evidence); or reduces the risk of developing any serious infection (one study, 89 participants, RR: 1.23, 95% CI 0.74 to 2.04, very low-quality evidence).A restrictive RBC transfusion policy may reduce the number of RBC transfusions per participant (two trials; 95 participants; mean difference (MD) -3.58, 95% CI -5.66 to -1.49, low-quality evidence). Evidence from NRSWe are uncertain whether the restrictive RBC transfusion strategy: reduces the risk of death within 100 days (one study, 84 participants, restrictive 1 death; liberal 1 death; very low-quality evidence); decreases the risk of clinically significant bleeding (one study, 84 participants, restrictive 3; liberal 8; very low-quality evidence); or decreases the number of RBC transfusions (adjusted for age, sex and acute myeloid leukaemia type geometric mean 1.25; 95% CI 1.07 to 1.47 - data analysis performed by the study authors)No NRS were found that looked at: quality of life; number of participants with any bleeding; serious infection; or length of hospital stay.No studies were found that looked at: adverse transfusion reactions; arterial or venous thromboembolic events; length of intensive care admission; or readmission to hospital.
Authors' conclusions: Findings from this review were based on four studies and 240 participants.There is low-quality evidence that a restrictive RBC transfusion policy reduces the number of RBC transfusions per participant. There is low-quality evidence that a restrictive RBC transfusion policy has little or no effect on: mortality at 30 to 100 days, bleeding, or hospital stay. This evidence is mainly based on adults with acute leukaemia who are having chemotherapy. Although, the two ongoing studies (530 participants) are due to be completed by January 2018 and will provide additional information for adults with haematological malignancies, we will not be able to answer this review's primary outcome. If we assume a mortality rate of 3% within 100 days we would need 1492 participants to have a 80% chance of detecting, as significant at the 5% level, an increase in all-cause mortality from 3% to 6%. Further RCTs are required in children.
Conflict of interest statement
Declarations of Interest Lise Estcourt is partly funded by NIHR Cochrane Programme Grant - Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest. Reem Malouf is partly funded by NIHR Cochrane Programme Grant - Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest. Marialena Trivella is partly funded by NIHR Cochrane Programme Grant - Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest. Sally Hopewell is partly funded by NIHR Cochrane Programme Grant - Safe and Appropriate Use of Blood Components. Award of this national government grant by NIHR does not lead to a conflict of interest. Dean A Fergusson: None known Mike Murphy: None known.
Figures












References
References to studies included in this review
De Zern 2016 {published data only}
-
- DeZern AE, Williams K, King K, Hand W, Levis M, Smith BD, et al. Liberal vs. restrictive transfusion thresholds in leukemia patients: a feasibility pilot study. The American Society of Hematology (ASH) the 57th Annual meeting & exposition Orlando. 2015.
-
- NCT02086773. Prospective randomized clinical feasibility study of red cell transfusion goals in patients with acute leukemias. clinicaltrials.gov/ct2/show/NCT02086773 (accessed 14 January 2016).
Jansen 2004 {published data only}
-
- Jansen AJ, Caljouw MA, Hop WC, Rhenen DJ, Schipperus MR. Feasibility of a restrictive red‐cell transfusion policy for patients treated with intensive chemotherapy for acute myeloid leukaemia. Transfusion Medicine 2004;14(1):33‐8. - PubMed
Robitaille 2013 {published data only}
-
- NCT00937053. Maintaining a higher level of haemoglobin: effect on the white cells after bone marrow transplantation in children. clinicaltrials.gov/ct2/show/NCT00937053 (accessed 14 January 2016).
-
- Robitaille N, Lacroix J, Alexandrov L, Clayton L, Cortier M, Schultz KR, et al. Excess of veno‐occlusive disease in a randomized clinical trial on a higher trigger for red blood cell transfusion after bone marrow transplantation: a Canadian blood and marrow transplant group trial. Biology of Blood and Marrow Transplantation 2013;19(3):468‐73. - PubMed
Webert 2008 {published data only}
-
- Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicentre pilot‐randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukaemia or stem cell transplantation . Transfusion 2008;48(1):81‐91. - PubMed
References to studies excluded from this review
Abels 1991 {published data only}
-
- Abels R, Gordon D, Nelson R, Krantz K, Ageeb M, Goon B, et al. Transfusion practice in advanced cancer patients. Blood 1991; Vol. 78:474a.
Almeida 2013 {published data only}
-
- Almeida JP, Galas F, Osawa E, Fukushima J, Moulin S, Park C, et al. Transfusion requirements in surgical oncology patients (TRISOP):a randomized, controlled trial. Critical Care 2013;17 Suppl 2:36410.1186/cc12302.
Bercovitz 2011 {published data only}
-
- Bercovitz RS, Dietz AC, Magid RN, Zantek ND, Smith AR, Quinones RR. What is the role of red blood cell transfusion threshold on number of transfusions in pediatric patients after hematopoietic stem cell transplant?. Blood 2011;118(21):1263.
Berger 2012 {published data only}
-
- Berger MD, Gerber B, Arn K, Senn O, Schanz U, Stussi G. Significant reduction of red blood cell transfusion requirements by changing from a double‐unit to a single‐unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica 2012;97(1):116‐22. - PMC - PubMed
Bruun 2011 {published data only}
-
- Bruun KH, Norgaard A, Johansson P, Daugaard G, Sorensen M. HaemOPtimal: Randomized feasibility study of the optimal haemoglobin trigger for red blood cell transfusion (RBC) of anaemic cancer patients (PTS) treated with chemotherapy (CT). Transfusion Alternatives in Transfusion Medicine 2011;12(1):19‐20.
Carson 2014 {published data only}
-
- Carson JL, Strair R. Transfusion strategies in hematologic and nonhematologic disease. Hematology, the American Society of Hematology Education Program 2014;5(1):548‐52. - PubMed
Holst 2013 {published data only}
ISRCTN26088319 {published data only}
-
- ISRCTN26088319. A trial looking at blood transfusions and quality of life in people with myelodysplastic syndrome (REDDS). controlled‐trials.com/ISRCTN26088319 (accessed 14 Jan 2016).
Lightdale 2012 {published data only}
-
- Lightdale JR, Randolph AG, Tran CM, Jiang H, Colon A, Houlahan K, et al. Impact of a conservative red blood cell transfusion strategy in children undergoing hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2012;18(5):813‐7. - PubMed
Mear 2014 {published data only}
-
- Mear JB, Chantepie S, Gac AC, Bazin A, Reman O. A restrictive strategy reduces the number of transfused packed red blood cells in allograft recipients. Blood 2014;124(21):5016.
NCT00202371 {published data only}
-
- NCT00202371. Transfusion effects in myelodysplastic patients: limiting exposure. clinicaltrials.gov/ct2/show/NCT00202371 (accessed 14 January 2016).
NTR2684 {published data only}
-
- NTR2684. Transfusion as supportive care for the improvement of the quality of life. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2684 (accessed 14 January 2016).
Paananen 2009 {published data only}
-
- Paananen P, Arola MO, Pelliniemi T, Salmi TT, Lahteenmaki PM. Evaluation of the effects of different transfusion trigger levels during the treatment of childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology/Oncology 2009;31:745‐9. - PubMed
Patil 2013 {published data only}
-
- Patil NR, Marques M, Mineishi S, Rudolph S, Vaughan W, Innis‐Shelton R, et al. A restrictive red cell transfusion approach does not adversely affect day 100 or 1 year survival in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation 2013;19:S268.
Prescott 2016 {published data only}
Yakymenko 2015 {published data only}
-
- NCT01116479. Optimal transfusion in anaemic cancer patients treated with chemotherapy (HaemOPtimal) (HaemOPtimal). https://clinicaltrials.gov/ct2/show/NCT01116479 Accessed 27 June 2016.
-
- Yakymenko D, Frandsen KB, Christensen IJ, Norgaard A, Johansson P, Daugaard G, et al. Haemoptimal: Randomized feasibility study of the optimal hemoglobin trigger for red blood cell transfusion to anemic cancer patients treated with chemotherapy. Supportive Care in Cancer 2015;23(Supp 1):S275. - PubMed
References to studies awaiting assessment
NCT02099669 {published data only}
-
- NCT02099669. Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC). clinicaltrials.gov/ct2/show/NCT02099669 (accessed 14 Jan 2016).
References to ongoing studies
Chantepie 2015 {published data only}
-
- NCT02461264. Randomized trial of two transfusion strategies in patients hospitalized for acute leukemia induction chemotherapy or hematopoietic stem cells with medico‐economic evaluation (1 VERSUS 2 CGR). https://clinicaltrials.gov/ct2/show/study/NCT02461264 ( accessed 28 January 2016).
Tay 2011 {published data only}
-
- NCT01237639. Study of red blood cell transfusion triggers in patients undergoing hematopoietic stem cell transplantation (TRIST). clinicaltrials.gov/ct2/show/NCT01237639 (accessed 14 January 2016).
Additional references
Adam 1942
-
- Adam RC, Lundy JS. Anesthesia in cases of poor risk. Some suggestions for decreasing the risk. Surgery, Gynecology & Obstetrics 1942;74:1011‐101.
Bearman 1988
-
- Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen‐related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology 1988;6(10):1562‐8. - PubMed
Beck 1998
-
- Beck C, Dubois J, Grignon A, Lacroix J, David M. Incidence and risk factors of catheter‐related deep vein thrombosis in a pediatric intensive care unit: a prospective study. Journal of Pediatrics 1998;133:237‐41. - PubMed
Blajchman 2005
-
- Blajchman MA. Transfusion‐related immunomodulation or TRIM: what does it mean clinically?. Hematology 2005;10 Suppl 1:208‐14. - PubMed
Bolton‐Maggs 2014
-
- Bolton‐Maggs PHB (Ed), Thomas D, Cohen H, Watt A, Poles D, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report (2014). http://www.shotuk.org/wp‐content/uploads/report‐2014.pdf (accessed 29 January 2016):8‐197.
Borkent‐Raven 2010
-
- Borkent‐Raven BA, Janssen MP, Poel CL, Schaasberg WP, Bonsel GJ, Hout BA. The PROTON study: profiles of blood product transfusion recipients in the Netherlands. Vox Sanguinis 2010;99(1):54‐64. - PubMed
Carson 2011
Carson 2012
-
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2012;157(1):49‐58. - PubMed
Carson 2013
Carson 2016
Cholette 2011
-
- Cholette JM, Rubenstein JS, Alfieris GM, Powers KS, Eaton M, Lerner NB. Children with single‐ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red‐cell transfusion strategy. Pediatric Critical Care Medicine 2011;12(1):39‐45. - PubMed
Colomo 2008
-
- Colomo A, Hernandez‐Gea V, Muniz‐Diaz E, Madoz P, Aracil C, Alvarez‐Urturi C, et al. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology 2008;48(S1):413A. [DOI: 10.1002/hep.22641] - DOI
Cook 1997
-
- Cook RJ, Lawless JF. Marginal analysis of recurrent events and a terminating event. Statistics in Medicine 1997;16(8):911‐24. - PubMed
CTCAE 2009
-
- National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0.3. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8... (accessed 23 July 2013):3‐194.
De Gast‐Bakker 2013
-
- Gast‐Bakker DH, Wilde RBP, Hazekamp MG, Sojak V, Zwaginga JJ, Wolterbeek R, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients:a randomized controlled trial. Intensive Care Medicine 2013;39:2011‐9. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statsitical Methods Group (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Docherty 2016
Fan 2014
-
- Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Archives of Gerontologyand Geriatrics 2014;59(1):181‐5. - PubMed
Fisher 1956
-
- Fisher MR, Topley ET. The illness of trauma. British Journal of Clinical Practice 1956;10(11):770‐6. - PubMed
Fortune 1987
-
- Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. Journal of Trauma 1987;27(3):243‐9. - PubMed
Gunn 2012
-
- Gunn S, Davis K, Ekert H, Ireland S, Thompson A, on behalf of the National Blood Authority (Australia). Patient blood management guidelines: module 3 medical. http://www.blood.gov.au/system/files/documents/pbm‐module3_0.pdf (accessed 17 February 2013); Vol. 1:36.
Hajjar 2010
-
- Hajjar LA, Vincent JL, Galas FRGB, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304(14):1559‐67. - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 1996
-
- Ho CH. The hemostatic effect of adequate red cell transfusion patients with anaemia and thrombocytopenia. Transfusion 1996;36(3):290. - PubMed
Ho 1998
-
- Ho CH. The hemostatic effect of packed red cell transfusion in patients with anemia. Transfusion 1998;38:1011‐4. - PubMed
Holst 2015
Holst 2016
-
- Holst LB. Benefits and harms of red blood cell transfusions in patients with septic shock in the Intensive Care Unit. Danish Medical Journal 2016;63(2):1‐18. - PubMed
Jairath 2015
-
- Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic,open‐label, cluster randomised feasibility trial.. Lancet 2015;386(9989):137‐44. - PubMed
Javadzadeh Shahshahani 2015
-
- Javadzadeh Shahshahani H, Hatami H, Meraat N, Savabieh S. Epidemiology of blood component recipients in hospitals of Yazd, Iran. Transfusion Medicine 2015;25(1):2‐7. - PubMed
Khorana 2008
Knight 2004
-
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anaemia in cancer: a systematic review of the literature. American Journal of Medicine 2004;116(7 Suppl 1):11‐26. - PubMed
Lacroix 2007
-
- Lacroix J, Hébert P, Hutchison J, Hume H, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine 2007;356(16):1609‐19. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Livio 1982
-
- Livio M, Gotti E, Marchesi D, Mecca G, Remuzzi G, Gaetano G. Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet 1982;320:1013‐5. - PubMed
McCelland 2010
-
- McCelland DBL, Pine E, Fraklin IM, for the EU Optimal Use of Blood Project Partners. Manual of Optimal Blood Use. Support for safe, clinically effective and efficient use of blood in Europe. http://www.optimalblooduse.eu (accessed 17 February 2013); Vol. 1:34.
Mendoza 1999
-
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186‐96. - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9, W64. - PubMed
Murphy 2011
-
- Murphy MF, Stanworth SJ, Yazer M. Transfusion practice and safety: current status and possibilities for improvement. Vox Sanguinis 2011;100(1):46‐59. - PubMed
Murphy 2015
-
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. New England Journal of Medicine 2015;372:997‐1008. - PubMed
NICE 2015
-
- National Institute for Health and Care Excellence. Blood transfusion. (NICE guideline 24.) 2015. www.nice.org.uk/guidance/NG24 ( Accessed 14 January 2016).
Nielsen 2014
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Quintó 2006
-
- Quintó L, Aponte JJ, Menéndez C, Sacarlal J, Aide P, Espasa M, et al. Relationship between haemoglobin and haematocrit in the definition of anaemia. Tropical Medicine and International Health 2006;11(8):1295‐302. - PubMed
Reeves 2011
-
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA, on behalf of the Cochrane Non‐Randomised Studies Methods Group. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Inverventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Review Manager (RevMan) 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2014
Rohde 2014
Salpeter 2014
-
- Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta‐analysis and systematic review. American Journal of Medicine 2014;127:124‐31. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH, on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
-
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tierney 2007
Tinegate 2016
-
- Tinegate H, Pendry K, Murphy M, Babra P, Grant‐Casey J, Hopkinson C, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139‐45. - PubMed
Valeri 1998
-
- Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has a sin of commission now become a sin of omission?. Transfusion 1998;38(6):602‐10. - PubMed
Vamvakas 2007
-
- Vamvakas EC, Blajchman MA. Transfusion‐related immunomodulation (TRIM): an update. Blood Reviews 2007;21(6):327‐48. - PubMed
Villanueva 2013
-
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New England Journal of Medicine 2013;368(1):11‐21. [10.1056/NEJMoa1211801.] - PubMed
Wells 2013
-
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 01 January 2013).
Whitaker 2015
-
- Whitaker BI, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. http://www.aabb.org/research/hemovigilance/bloodsurvey/Pages/default.aspx 2015. - PubMed
WHO 2015
-
- World Health Organizaition (WHO). Blood safety and availability, fact sheet N°279. http://www.who.int/mediacentre/factsheets/fs279/en/ (accessed 01 February 2016).
Wu 2011
-
- Wu JF, Guan XD, Chen J, Ou‐Yang B. A randomized, controlled clinical trial of transfusion of requirement in patients after orthotopic liver transplantation. ESICM LIVES 2011, 24th Annual Congress 2011;37:Abstract.
Zygun 2009
-
- Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Critical Care Medicine 2009;37(4):1074‐8. - PubMed
References to other published versions of this review
Butler 2014
-
- Butler C, Tay J, Doree C, Brunskill SJ, Trivella M, Fergusson DA, et al. Restrictive versus liberal red blood cell transfusion strategies for patients with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011305] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

