Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment
- PMID: 28128852
- PMCID: PMC5573922
- DOI: 10.1002/hep.29081
Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment
Erratum in
-
Correction.Hepatology. 2017 Nov;66(5):1708. doi: 10.1002/hep.29551. Hepatology. 2017. PMID: 29053199 Free PMC article. No abstract available.
Abstract
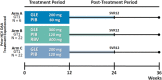
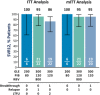
Although direct-acting antiviral (DAA) therapies for chronic hepatitis C virus (HCV) infection have demonstrated high rates of sustained virologic response, virologic failure may still occur, potentially leading to the emergence of viral resistance, which can decrease the effectiveness of subsequent treatment. Treatment options for patients who failed previous DAA-containing regimens, particularly those with nonstructural protein 5A inhibitors, are limited and remain an area of unmet medical need. This phase 2, open-label study (MAGELLAN-1) evaluated the efficacy and safety of glecaprevir (GLE) + pibrentasvir (PIB) ± ribavirin (RBV) in HCV genotype 1-infected patients with prior virologic failure to HCV DAA-containing therapy. A total of 50 patients without cirrhosis were randomized to three arms: 200 mg GLE + 80 mg PIB (arm A), 300 mg GLE + 120 mg PIB with 800 mg once-daily RBV (arm B), or 300 mg GLE + 120 mg PIB without RBV (arm C). By intent-to-treat analysis, sustained virologic response at posttreatment week 12 was achieved in 100% (6/6, 95% confidence interval 61-100), 95% (21/22, 95% confidence interval 78-99), and 86% (19/22, 95% confidence interval 67-95) of patients in arms A, B, and C, respectively. Virologic failure occurred in no patients in arm A and in 1 patient each in arms B and C (two patients were lost to follow-up in arm C). The majority of adverse events were mild in severity; no serious adverse events related to study drug and no relevant laboratory abnormalities in alanine aminotransferase, total bilirubin, or hemoglobin were observed.
Conclusion: The combination of GLE and PIB was highly efficacious and well tolerated in patients with HCV genotype 1 infection and prior failure of DAA-containing therapy; RBV coadministration did not improve efficacy. (Hepatology 2017;66:389-397).
© 2017 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures
References
-
- Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(1 Suppl.):S45‐S57. - PubMed
-
- Asselah T, Boyer N, Saadoun D, Martinot‐Peignoux M, Marcellin P. Direct‐acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN‐free treatment and future perspectives. Liver Int 2016;36(Suppl. 1):47‐57. - PubMed
-
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014;146:1176‐1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

