Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma
- PMID: 28129122
- PMCID: PMC5363293
- DOI: 10.1016/j.ymthe.2016.10.020
Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma
Abstract
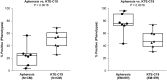
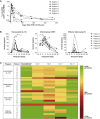
Outcomes for patients with refractory diffuse large B cell lymphoma (DLBCL) are poor. In the multicenter ZUMA-1 phase 1 study, we evaluated KTE-C19, an autologous CD3ζ/CD28-based chimeric antigen receptor (CAR) T cell therapy, in patients with refractory DLBCL. Patients received low-dose conditioning chemotherapy with concurrent cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days followed by KTE-C19 at a target dose of 2 × 106 CAR T cells/kg. The incidence of dose-limiting toxicity (DLT) was the primary endpoint. Seven patients were treated with KTE-C19 and one patient experienced a DLT of grade 4 cytokine release syndrome (CRS) and neurotoxicity. Grade ≥3 CRS and neurotoxicity were observed in 14% (n = 1/7) and 57% (n = 4/7) of patients, respectively. All other KTE-C19-related grade ≥3 events resolved within 1 month. The overall response rate was 71% (n = 5/7) and complete response (CR) rate was 57% (n = 4/7). Three patients have ongoing CR (all at 12+ months). CAR T cells demonstrated peak expansion within 2 weeks and continued to be detectable at 12+ months in patients with ongoing CR. This regimen of KTE-C19 was safe for further study in phase 2 and induced durable remissions in patients with refractory DLBCL.
Keywords: CAR T; CD19; KTE-C19; diffuse large B cell lymphoma; refractory NHL.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Expanding Accessibility to CD19-CAR T Cells: Commercializing a "Boutique" Therapy.Mol Ther. 2017 Jan 4;25(1):8-9. doi: 10.1016/j.ymthe.2016.12.002. Epub 2017 Jan 4. Mol Ther. 2017. PMID: 28129132 Free PMC article. No abstract available.
References
-
- Chaganti S., Illidge T., Barrington S., Mckay P., Linton K., Cwynarski K., McMillan A., Davies A., Stern S., Peggs K., British Committee for Standards in Haematology Guidelines for the management of diffuse large B-cell lymphoma. Br. J. Haematol. 2016;174:43–56. - PubMed
-
- Sehn L.H., Gascoyne R.D. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. - PubMed
-
- Crump M., Kuruvilla J., Couban S., MacDonald D.A., Kukreti V., Kouroukis C.T., Rubinger M., Buckstein R., Imrie K.R., Federico M. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J. Clin. Oncol. 2014;32:3490–3496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

