HIV Drug Resistance Mutations in Non-B Subtypes After Prolonged Virological Failure on NNRTI-Based First-Line Regimens in Sub-Saharan Africa
- PMID: 28129253
- PMCID: PMC5427983
- DOI: 10.1097/QAI.0000000000001285
HIV Drug Resistance Mutations in Non-B Subtypes After Prolonged Virological Failure on NNRTI-Based First-Line Regimens in Sub-Saharan Africa
Abstract
Objective: To determine drug resistance mutation (DRM) patterns in a large cohort of patients failing nonnucleoside reverse transcriptase inhibitor (NNRTI)-based first-line antiretroviral therapy regimens in programs without routine viral load (VL) monitoring and to examine intersubtype differences in DRMs.
Design: Sequences from 787 adults/adolescents who failed an NNRTI-based first-line regimen in 13 clinics in Uganda, Kenya, Zimbabwe, and Malawi were analyzed. Multivariable logistic regression was used to determine the association between specific DRMs and Stanford intermediate-/high-level resistance and factors including REGA subtype, first-line antiretroviral therapy drugs, CD4, and VL at failure.
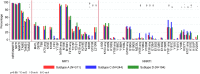
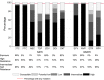
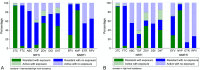
Results: The median first-line treatment duration was 4 years (interquartile range 30-43 months); 42% of participants had VL ≥100,000 copies/mL and 63% participants had CD4 <100 cells/mm. Viral subtype distribution was A1 (40%; Uganda and Kenya), C (31%; Zimbabwe and Malawi), and D (25%; Uganda and Kenya), and recombinant/unclassified (5%). In general, DRMs were more common in subtype-C than in subtype-A and/or subtype-D (nucleoside reverse transcriptase inhibitor mutations K65R and Q151M; NNRTI mutations E138A, V106M, Y181C, K101E, and H221Y). The presence of tenofovir resistance was similar between subtypes [P (adjusted) = 0.32], but resistance to zidovudine, abacavir, etravirine, or rilpivirine was more common in subtype-C than in subtype-D/subtype-A [P (adjusted) < 0.02].
Conclusions: Non-B subtypes differ in DRMs at first-line failure, which impacts on residual nucleoside reverse transcriptase inhibitor and NNRTI susceptibility. In particular, higher rates of etravirine and rilpivirine resistance in subtype-C may limit their potential utility in salvage regimens.
Conflict of interest statement
C.K., J.T., I.N., A.H., E.N., C.W., I.M., J.J.v.O., K.W.-K., P.J.E., P.M., A.S.W. and N.I.P. report grants from EDCTP, non-financial support from AbbVie, Janssen laboratories, Abbott Laboratories and Gilead, grants and non-financial support from GSK/ViiV and Merck, grants from WHO, non-financial support from Gilead during the conduct of the study. A.S.W. reports money paid to institution from Janssen and Gilead Sciences outside the submitted work. N.I.P. reports personal fees from AbbVie, Janssen and Roche outside the submitted work. S.B. reports no conflicts.
Figures

References
-
- Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. - PubMed
-
- World Health Organisation. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organisation; 2016. - PubMed
-
- Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

