Parenteral and enteral nutrition in surgical critical care: Plasma metabolomics demonstrates divergent effects on nitrogen, fatty-acid, ribonucleotide, and oxidative metabolism
- PMID: 28129265
- PMCID: PMC5360523
- DOI: 10.1097/TA.0000000000001381
Parenteral and enteral nutrition in surgical critical care: Plasma metabolomics demonstrates divergent effects on nitrogen, fatty-acid, ribonucleotide, and oxidative metabolism
Abstract
Background: Artificial nutrition support is central to the care of critically ill patients and is primarily provided enterally (EN). There are circumstances when parenteral nutrition (PN) is considered necessary. We are uncertain how each of these approaches confer clinical benefits beyond simply providing calories. We sought to better understand how each of these techniques influence metabolism in critically ill patients using a broad-based metabolomics approach. Metabolic responses to EN and PN may differ in ways that could help us understand how to optimize use of these therapies.
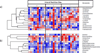
Methods: We prospectively enrolled subjects over 7 months in 2015 at an urban, Level I trauma center. Subjects were included before starting either EN or PN during their inpatient admission. Plasma samples were obtained between 1 and 12 hours before initiation of artificial nutrition, and 3 and 7 days later. All samples were analyzed with liquid chromatography/mass spectrometry-based metabolomics. Differences in metabolite concentrations were assessed via principal component analyses and multiple linear regression.
Results: We enrolled 30 subjects. Among the critically ill subjects, 10 received EN and 10 received PN. In subjects receiving EN, amino acid and urea cycle metabolites (citrulline, p = 0.04; ornithine, p = 0.05) increased, as did ribonucleic acid metabolites (uridine, p = 0.04; cysteine, 0 = 0.05; oxypurinol, p = 0.04). Oxidative stress decreased over time (increased betaine, p = 0.05; decreased 4-pyridoxic acid, p = 0.04). In subjects receiving PN, amino acid concentrations increased over time (taurine, p = 0.04; phenylalanine, p = 0.05); omega 6 and omega 3 fatty acid concentrations decreased over time (p = 0.05 and 0.03, respectively).
Conclusion: EN was associated with amino acid repletion, urea cycle upregulation, restoration of antioxidants, and increasing ribonucleic acid synthesis. Parenteral nutrition was associated with increased amino acid concentrations, but did not influence protein metabolism or antioxidant repletion. This suggests that parenteral amino acids are used less effectively than those given enterally. The biomarkers reported in this study may be useful in guiding nutrition therapy for critically ill patients.
Level of evidence: Therapeutic study, level III; prognostic study, level II.
Conflict of interest statement
Figures
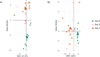





Similar articles
-
When early enteral feeding is not possible in critically ill patients: results of a multicenter observational study.JPEN J Parenter Enteral Nutr. 2011 Mar;35(2):160-8. doi: 10.1177/0148607110381405. JPEN J Parenter Enteral Nutr. 2011. PMID: 21378245
-
Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients.JPEN J Parenter Enteral Nutr. 2003 Sep-Oct;27(5):355-73. doi: 10.1177/0148607103027005355. JPEN J Parenter Enteral Nutr. 2003. PMID: 12971736
-
Impact of withholding early parenteral nutrition completing enteral nutrition in pediatric critically ill patients (PEPaNIC trial): study protocol for a randomized controlled trial.Trials. 2015 May 1;16:202. doi: 10.1186/s13063-015-0728-8. Trials. 2015. PMID: 25927936 Free PMC article. Clinical Trial.
-
Parenteral nutrition: never say never.Crit Care. 2015;19 Suppl 3(Suppl 3):S5. doi: 10.1186/cc14723. Epub 2015 Dec 18. Crit Care. 2015. PMID: 26728859 Free PMC article. Review.
-
The physiologic response and associated clinical benefits from provision of early enteral nutrition.Nutr Clin Pract. 2009 Jun-Jul;24(3):305-15. doi: 10.1177/0884533609335176. Nutr Clin Pract. 2009. PMID: 19483060 Review.
Cited by
-
Impact of the route of nutrition on gut mucosa in ventilated adults with shock: an ancillary of the NUTRIREA-2 trial.Intensive Care Med. 2019 Jul;45(7):948-956. doi: 10.1007/s00134-019-05649-3. Epub 2019 May 29. Intensive Care Med. 2019. PMID: 31143999 Clinical Trial.
-
Metabolomics and Precision Medicine in Trauma: The State of the Field.Shock. 2018 Jul;50(1):5-13. doi: 10.1097/SHK.0000000000001093. Shock. 2018. PMID: 29280924 Free PMC article.
-
A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox.Metabolites. 2021 Aug 20;11(8):552. doi: 10.3390/metabo11080552. Metabolites. 2021. PMID: 34436492 Free PMC article.
-
Targeted metabolomics reveals plasma biomarkers and metabolic alterations of the aging process in healthy young and older adults.Geroscience. 2023 Dec;45(6):3131-3146. doi: 10.1007/s11357-023-00823-4. Epub 2023 May 17. Geroscience. 2023. PMID: 37195387 Free PMC article.
-
Plasma metabolomics of early parenteral nutrition followed with enteral nutrition in pancreatic surgery patients.Sci Rep. 2019 Dec 11;9(1):18846. doi: 10.1038/s41598-019-55440-z. Sci Rep. 2019. PMID: 31827206 Free PMC article.
References
-
- Singer P, Pichard C. Reconciling divergent results of the latest parenteral nutrition studies in the ICU. Curr Op Clin Nutr. 2013;16(2):187–193. - PubMed
-
- Huynh D, Chapman MJ, Nguyen NQ. Nutrition support in the critically ill. Curr Op Gastroenterol. 2013;29(2):208–215. - PubMed
-
- Singer P, Anbar R, Cohen J, Sharpio H, Shalita-Chesner M, Lev S, Grozovski E, Theilla M, Frishman S, Madar Z. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Int Care Med. 2011;37(4):601–609. - PubMed
-
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, Vale LD, Battison CG, Jenkinson DJ, Cook JA. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

