Meneco, a Topology-Based Gap-Filling Tool Applicable to Degraded Genome-Wide Metabolic Networks
- PMID: 28129330
- PMCID: PMC5302834
- DOI: 10.1371/journal.pcbi.1005276
Meneco, a Topology-Based Gap-Filling Tool Applicable to Degraded Genome-Wide Metabolic Networks
Abstract
Increasing amounts of sequence data are becoming available for a wide range of non-model organisms. Investigating and modelling the metabolic behaviour of those organisms is highly relevant to understand their biology and ecology. As sequences are often incomplete and poorly annotated, draft networks of their metabolism largely suffer from incompleteness. Appropriate gap-filling methods to identify and add missing reactions are therefore required to address this issue. However, current tools rely on phenotypic or taxonomic information, or are very sensitive to the stoichiometric balance of metabolic reactions, especially concerning the co-factors. This type of information is often not available or at least prone to errors for newly-explored organisms. Here we introduce Meneco, a tool dedicated to the topological gap-filling of genome-scale draft metabolic networks. Meneco reformulates gap-filling as a qualitative combinatorial optimization problem, omitting constraints raised by the stoichiometry of a metabolic network considered in other methods, and solves this problem using Answer Set Programming. Run on several artificial test sets gathering 10,800 degraded Escherichia coli networks Meneco was able to efficiently identify essential reactions missing in networks at high degradation rates, outperforming the stoichiometry-based tools in scalability. To demonstrate the utility of Meneco we applied it to two case studies. Its application to recent metabolic networks reconstructed for the brown algal model Ectocarpus siliculosus and an associated bacterium Candidatus Phaeomarinobacter ectocarpi revealed several candidate metabolic pathways for algal-bacterial interactions. Then Meneco was used to reconstruct, from transcriptomic and metabolomic data, the first metabolic network for the microalga Euglena mutabilis. These two case studies show that Meneco is a versatile tool to complete draft genome-scale metabolic networks produced from heterogeneous data, and to suggest relevant reactions that explain the metabolic capacity of a biological system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

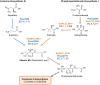

References
-
- Thiele I, Palsson BO. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nature Protocols. 2010. January;5(1):93–121. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3125167&tool=p.... 10.1038/nprot.2009.203 - DOI - PMC - PubMed
-
- Henry CS, DeJongh M, Best AA, Frybarger PM, Linsay B, Stevens RL. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nature Biotechnology. 2010. August;28(9):977–982. Available from: http://www.nature.com/doifinder/10.1038/nbt.1672. 10.1038/nbt.1672 - DOI - DOI - PubMed
-
- Agren R, Liu L, Shoaie S, Vongsangnak W, Nookaew I, Nielsen J. The RAVEN Toolbox and Its Use for Generating a Genome-scale Metabolic Model for Penicillium chrysogenum. PLoS Comput Biol. 2013. 03;9(3):e1002980 Available from: http://dx.doi.org/10.1371%2Fjournal.pcbi.1002980. - PMC - PubMed
-
- Vallenet D, Belda E, Calteau A, Cruveiller S, Engelen S, Lajus A, et al. MicroScope—an integrated microbial resource for the curation and comparative analysis of genomic and metabolic data. Nucleic Acids Research. 2013;41(D1):D636–D647. Available from: http://nar.oxfordjournals.org/content/41/D1/D636.abstract. 10.1093/nar/gks1194 - DOI - PMC - PubMed
-
- Karp PD, Paley S, Romero P. The Pathway Tools software. Bioinformatics. 2002. July;18(Suppl 1):S225–S232. Available from: http://bioinformatics.oxfordjournals.org/cgi/doi/10.1093/bioinformatics/.... 10.1093/bioinformatics/18.suppl_1.S225 - DOI - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

