A Novel Positron Emission Tomography (PET) Approach to Monitor Cardiac Metabolic Pathway Remodeling in Response to Sunitinib Malate
- PMID: 28129334
- PMCID: PMC5271313
- DOI: 10.1371/journal.pone.0169964
A Novel Positron Emission Tomography (PET) Approach to Monitor Cardiac Metabolic Pathway Remodeling in Response to Sunitinib Malate
Abstract
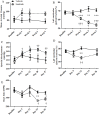
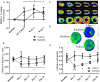
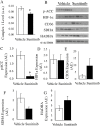
Sunitinib is a tyrosine kinase inhibitor approved for the treatment of multiple solid tumors. However, cardiotoxicity is of increasing concern, with a need to develop rational mechanism driven approaches for the early detection of cardiac dysfunction. We sought to interrogate changes in cardiac energy substrate usage during sunitinib treatment, hypothesising that these changes could represent a strategy for the early detection of cardiotoxicity. Balb/CJ mice or Sprague-Dawley rats were treated orally for 4 weeks with 40 or 20 mg/kg/day sunitinib. Cardiac positron emission tomography (PET) was implemented to investigate alterations in myocardial glucose and oxidative metabolism. Following treatment, blood pressure increased, and left ventricular ejection fraction decreased. Cardiac [18F]-fluorodeoxyglucose (FDG)-PET revealed increased glucose uptake after 48 hours. [11C]Acetate-PET showed decreased myocardial perfusion following treatment. Electron microscopy revealed significant lipid accumulation in the myocardium. Proteomic analyses indicated that oxidative metabolism, fatty acid β-oxidation and mitochondrial dysfunction were among the top myocardial signalling pathways perturbed. Sunitinib treatment results in an increased reliance on glycolysis, increased myocardial lipid deposition and perturbed mitochondrial function, indicative of a fundamental energy crisis resulting in compromised myocardial energy metabolism and function. Our findings suggest that a cardiac PET strategy may represent a rational approach to non-invasively monitor metabolic pathway remodeling following sunitinib treatment.
Conflict of interest statement
There are no competing interests associated with the publication of this manuscript and the commercial affiliations do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- FDA. Sutent Medication Guide (http://www.fda.gov/downloads/drugs/drugsafety/ucm219111.pdf) [PDF]. www.FDA.gov: Pfizer; 2013 [updated August 2013; cited 2014 13th May]. http://www.fda.gov/downloads/drugs/drugsafety/ucm219111.pdf.
-
- Clinicaltrials.gov. List results, search of sunitinib http://www.clinicaltrials.gov/ct2/results?termsunitinib 2014 [cited 20/10/2014]. http://www.clinicaltrials.gov/ct2/results?term=sunitinib&recr=Open.
-
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

