Crystal Structure of an LSD-Bound Human Serotonin Receptor
- PMID: 28129538
- PMCID: PMC5289311
- DOI: 10.1016/j.cell.2016.12.033
Crystal Structure of an LSD-Bound Human Serotonin Receptor
Abstract
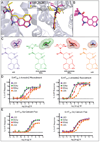
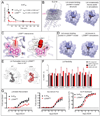
The prototypical hallucinogen LSD acts via serotonin receptors, and here we describe the crystal structure of LSD in complex with the human serotonin receptor 5-HT2B. The complex reveals conformational rearrangements to accommodate LSD, providing a structural explanation for the conformational selectivity of LSD's key diethylamide moiety. LSD dissociates exceptionally slow from both 5-HT2BR and 5-HT2AR-a major target for its psychoactivity. Molecular dynamics (MD) simulations suggest that LSD's slow binding kinetics may be due to a "lid" formed by extracellular loop 2 (EL2) at the entrance to the binding pocket. A mutation predicted to increase the mobility of this lid greatly accelerates LSD's binding kinetics and selectively dampens LSD-mediated β-arrestin2 recruitment. This study thus reveals an unexpected binding mode of LSD; illuminates key features of its kinetics, stereochemistry, and signaling; and provides a molecular explanation for LSD's actions at human serotonin receptors. PAPERCLIP.
Keywords: GPCR; crystallography; hallucinogens; serotonin receptor; structure-function.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
A Receptor on Acid.Cell. 2017 Jan 26;168(3):339-341. doi: 10.1016/j.cell.2017.01.012. Cell. 2017. PMID: 28129534 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

