Interspecies Chimerism with Mammalian Pluripotent Stem Cells
- PMID: 28129541
- PMCID: PMC5679265
- DOI: 10.1016/j.cell.2016.12.036
Interspecies Chimerism with Mammalian Pluripotent Stem Cells
Abstract
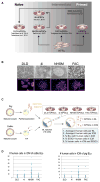
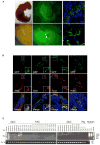
Interspecies blastocyst complementation enables organ-specific enrichment of xenogenic pluripotent stem cell (PSC) derivatives. Here, we establish a versatile blastocyst complementation platform based on CRISPR-Cas9-mediated zygote genome editing and show enrichment of rat PSC-derivatives in several tissues of gene-edited organogenesis-disabled mice. Besides gaining insights into species evolution, embryogenesis, and human disease, interspecies blastocyst complementation might allow human organ generation in animals whose organ size, anatomy, and physiology are closer to humans. To date, however, whether human PSCs (hPSCs) can contribute to chimera formation in non-rodent species remains unknown. We systematically evaluate the chimeric competency of several types of hPSCs using a more diversified clade of mammals, the ungulates. We find that naïve hPSCs robustly engraft in both pig and cattle pre-implantation blastocysts but show limited contribution to post-implantation pig embryos. Instead, an intermediate hPSC type exhibits higher degree of chimerism and is able to generate differentiated progenies in post-implantation pig embryos.
Keywords: CRISPR-Cas9; human naïve pluripotent stem cells; human-cattle chimeric embryo; human-pig chimeric embryo; interspecies blastocyst complementation; interspecies chimera; organ and tissue generation; pluripotent stem cells; zygote genome editing.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, González F, Vassena R, Bilić J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. - PubMed
-
- Abeydeera LR. In vitro production of embryos in swine. Theriogenology. 2002;57:256–273. - PubMed
-
- De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, Hochedlinger K, Jaenisch R, Lee S, Leitch HG, et al. Hallmarks of pluripotency. Nature. 2015;525:469–478. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

