Differential regulation of cellular functions by the C-termini of transmembrane 4 L six family proteins in 2- or 3-dimensional environment
- PMID: 28129652
- PMCID: PMC5355095
- DOI: 10.18632/oncotarget.14809
Differential regulation of cellular functions by the C-termini of transmembrane 4 L six family proteins in 2- or 3-dimensional environment
Abstract
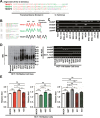
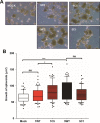
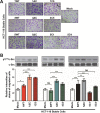
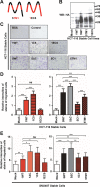
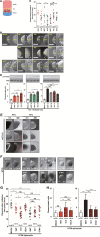
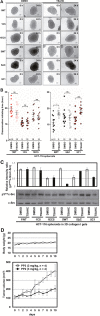
The transmembrane 4 L six family proteins TM4SF1, TM4SF4, and TM4SF5 share 40-50% overall sequence identity, but their C-terminus identity is limited. It may be likely that the C-termini of the members are important and unique for own regulatory functions. We thus examined how the TM4SF5 C-terminus affected cellular functions differentially from other family members. Using colon cancer cells expressing wildtype (WT), C-terminus-deleted, or chimeric mutants, diverse cellular functions were explored in 2-dimensional (2D) and 3-dimensional (3D) condition. The C-termini of the proteins were relatively comparable with respect to 2D cell proliferation, although each C-terminal-deletion mutant exhibited increased proliferation relative to the WT. Using chimeric constructs, we found that the TM4SF5 C-terminus was critical for regulating the diverse metastatic functions of TM4SF5, and could positively replace the C-termini of other family members. Replacement of the TM4SF1 or TM4SF4 C-terminus with that of TM4SF5 increased spheroids growth, transwell migration, and invasive dissemination from spheroids in 3D collagen gels. TM4SF5-mediated effects required its extracellular loop 2 linked to the C-terminus via the transmembrane domain 4, with causing c-Src activation. Altogether, the C-terminus of TM4SF5 appears to mediate pro-migratory roles, depending on a structural relay from the second extracellular loop to the C-terminus.
Keywords: 3D cell culture; migration; proliferation; spheroids; transmembrane 4 L six family.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. - PubMed
-
- Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127(Pt 17):3641–3648. - PubMed
-
- Lee JW. Transmembrane 4 L Six Family Member 5 (TM4SF5)-Mediated Epithelial-Mesenchymal Transition in Liver Diseases. Int Rev Cell Mol Biol. 2015;319:141–163. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

