Investigating the mechanism of ADP-forming acetyl-CoA synthetase from the protozoan parasite Entamoeba histolytica
- PMID: 28129670
- PMCID: PMC5330840
- DOI: 10.1002/1873-3468.12573
Investigating the mechanism of ADP-forming acetyl-CoA synthetase from the protozoan parasite Entamoeba histolytica
Abstract
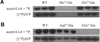
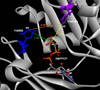
ADP-forming acetyl-CoA synthetase (ACD) catalyzes the interconversion of acetyl-CoA and acetate. The related succinyl-CoA synthetase follows a three-step mechanism involving a single phosphoenzyme, but a novel four-step mechanism with two phosphoenzyme intermediates was proposed for Pyrococcus ACD. Characterization of enzyme variants of Entamoeba ACD in which the two proposed phosphorylated His residues were individually altered revealed that only His252 is essential for enzymatic activity. Analysis of variants altered at two residues proposed to interact with the phosphohistidine loop that swings between distinct parts of the active site are consistent with a mechanism involving a single phosphoenzyme intermediate. Our results suggest ACDs with different subunit structures may employ slightly different mechanisms to bridge the span between active sites I and II.
Keywords: ATP; Entamoeba; acetate; acetyl-CoA; acetyl-CoA synthetase; energy metabolism.
© 2017 Federation of European Biochemical Societies.
Figures


Similar articles
-
Biochemical and kinetic characterization of the recombinant ADP-forming acetyl coenzyme A synthetase from the amitochondriate protozoan Entamoeba histolytica.Eukaryot Cell. 2014 Dec;13(12):1530-7. doi: 10.1128/EC.00192-14. Epub 2014 Oct 10. Eukaryot Cell. 2014. PMID: 25303954 Free PMC article.
-
Reaction mechanism and structural model of ADP-forming Acetyl-CoA synthetase from the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a second active site histidine residue.J Biol Chem. 2008 May 30;283(22):15409-18. doi: 10.1074/jbc.M710218200. Epub 2008 Mar 27. J Biol Chem. 2008. PMID: 18372246 Free PMC article.
-
Purification and characterization of two reversible and ADP-dependent acetyl coenzyme A synthetases from the hyperthermophilic archaeon Pyrococcus furiosus.J Bacteriol. 1996 Oct;178(20):5897-903. doi: 10.1128/jb.178.20.5897-5903.1996. J Bacteriol. 1996. PMID: 8830684 Free PMC article.
-
An n.m.r. probe of succinyl-coenzyme A synthetase: subunit interactions and the mechanism of action.Biochem Soc Trans. 1983 Jun;11(3):315-23. doi: 10.1042/bst0110315. Biochem Soc Trans. 1983. PMID: 6347742 Review. No abstract available.
-
Acetate formation in the energy metabolism of parasitic helminths and protists.Int J Parasitol. 2010 Mar 15;40(4):387-97. doi: 10.1016/j.ijpara.2009.12.006. Epub 2010 Jan 18. Int J Parasitol. 2010. PMID: 20085767 Review.
References
-
- Sanchez LB, Galperin MY, Muller M. Acetyl-CoA synthetase from the amitochondriate eukaryote Giardia lamblia belongs to the newly recognized superfamily of acyl-CoA synthetases (Nucleoside diphosphate-forming) J. Biol. Chem. 2000;275:5794–5803. - PubMed
-
- Brasen C, Schonheit P. Unusual ADP-forming acetyl-coenzyme A synthetases from the mesophilic halophilic euryarchaeon Haloarcula marismortui and from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum . Archives Microbiol. 2004;182:277–287. - PubMed
-
- Schafer T, Schonheit P. Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Acetate formation from acetyl-CoA and ATP synthesis are catalyzed by an acetyl-CoA synthetase (ADP-forming) Archives Microbiol. 1991;155:366–377.
-
- Schafer T, Selig M, Schonheit P. Acetyl-CoA synthetase (ADP-forming) in archaea, a novel enzyme involved in acetate formation and ATP synthesis. Archives Microbiol. 1993;159:72–83.
-
- Schmidt M, Schonheit P. Acetate formation in the photoheterotrophic bacterium Chloroflexus aurantiacus involves an archaeal type ADP-forming acetyl-CoA synthetase isoenzyme I. FEMS Microbiol. Lett. 2013;349:171–179. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

