A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART
- PMID: 28129756
- PMCID: PMC5273845
- DOI: 10.1186/s12888-017-1208-3
A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART
Abstract
Background: Interventions for alcohol use disorders (AUDs) in HIV infected individuals have been primarily targeted at HIV risk reduction and improved antiretroviral treatment adherence. However, reduction in alcohol use is an important goal. Alcohol use affects other key factors that may influence treatment course and outcome. In this study the authors aim to administer an adapted intervention for AUDs to reduce alcohol use in people living with HIV/AIDS (PLWHA).
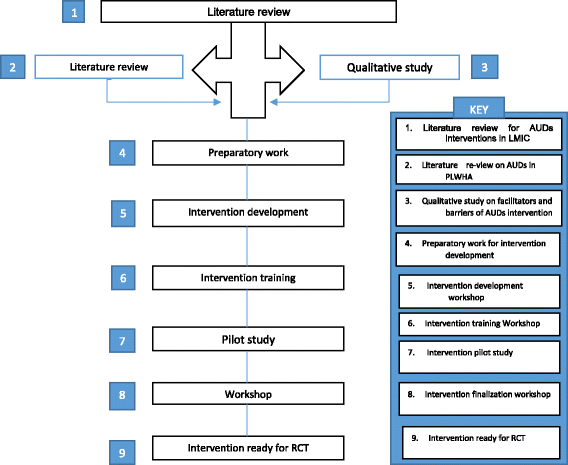
Methods: This study is a cluster randomised controlled trial at 16 HIV care clinics. A motivational interviewing and cognitive behavioural therapy based intervention for AUDs, developed through adaptation and piloted in Zimbabwe, will be administered to PLWHA with AUDs recruited at HIV clinics. The intervention will be administered over 16 sessions at 8 HIV clinics. This intervention will be compared with an equal attention control in the form of the World Health Organization Mental Health Gap Action Programme (WHO mhGAP) guide, adapted for the Zimbabwean context. General function, quality of life, and adherence to highly active antiretroviral treatment (HAART) will be secondary outcomes. Booster sessions will be administered to both groups at 3 and 6 months respectively. The primary outcome measure will be the Alcohol Use Disorder Identification Test (AUDIT) score. The World Health Organisation Disability Assessment Schedule 2.0 (WHODAS 2.0), World Health Organisation Quality of Life (WHOQoL) HIV, viral load, and CD4 counts will be secondary outcome measures. Outcome assessments will be administered at baseline, 3, 6, and 12 months. Moderating factors such as perceived social support, how people cope with difficult situations and post-traumatic exposure and experience will be assessed at baseline. Trained research assistants will recruit participants. The outcome assessors who will be trained in administering the outcome and moderating tools will be blinded to the treatment arms allocated to the participants. However, the principal investigator, participants and intervention staff will be unblinded. Data will be analysed using STATA Version 14. Primary and secondary outcomes will be measured at four time points that is; at baseline, 3, 6, and 12 months respectively. All participants will be included in the analysis of primary and secondary outcome measures. The mean AUDIT scores will be compared between groups using student t-tests. Multilevel logistic regression analysis will be performed for binominal variables and multilevel linear regression for continuous variables. Descriptive statistics will be computed for baseline and follow-up assessments.
Discussion: The study will be the first to address problematic alcohol use in PLWHA in Zimbabwe. It seeks to use local resources in delivering a modified, brief, evidence-based, and culturally contextualised intervention. The study results will determine the effectiveness of adapting psychological interventions for AUDs in HIV infected adults using a task-sharing framework.
Trial registration: Pan African Clinical Trial Registry, PACTR201509001211149 . Registered 22 July 2015.
Keywords: Alcohol use disorders; Cognitive behavioural therapy; Intervention; Motivational interviewing; Zimbabwe.
Figures
References
-
- Organization WH . International guide for monitoring alcohol consumption and related harm. 2000.
-
- Organization WH . Global status report on alcohol 2004. 2004.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous