Manubrium-limited ministernotomy versus conventional sternotomy for aortic valve replacement (MAVRIC): study protocol for a randomised controlled trial
- PMID: 28129780
- PMCID: PMC5273792
- DOI: 10.1186/s13063-016-1768-4
Manubrium-limited ministernotomy versus conventional sternotomy for aortic valve replacement (MAVRIC): study protocol for a randomised controlled trial
Abstract
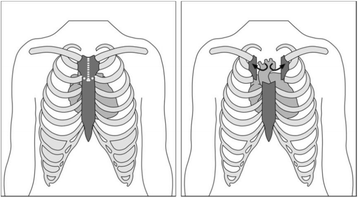
Background: Aortic valve replacement is one of the most common cardiac surgical procedures performed worldwide. Conventional aortic valve replacement surgery is performed via a median sternotomy; the sternum is divided completely from the sternal notch to the xiphisternum. Minimally invasive aortic valve replacement, using a new technique called manubrium-limited ministernotomy, divides only the manubrium from the sternal notch to 1 cm below the manubrio-sternal junction. More than one third of patients undergoing conventional sternotomy develop clinically significant bleeding requiring post-operative red blood cell transfusion. Case series data suggest a potentially clinically significant difference in red blood cell transfusion requirements between the two techniques. Given the implications for National Health Service resources and patient outcomes, a definitive trial is needed.
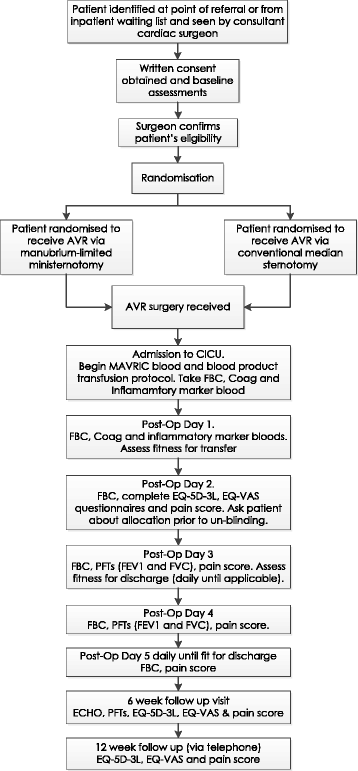
Methods/design: This is a single-centre, single-blind, randomised controlled trial comparing aortic valve replacement surgery using manubrium-limited ministernotomy (intervention) and conventional median sternotomy (usual care). Two hundred and seventy patients will be randomised in a 1:1 ratio between the intervention and control arms, stratified by baseline logistic EuroSCORE and haemoglobin value. Patients will be followed for 12 weeks from discharge following their index operation. The primary outcome is the proportion of patients who receive a red blood cell transfusion post-operatively within 7 days of surgery. Secondary outcomes include red blood cell and blood product transfusions, blood loss, re-operation rates, sternal wound pain, quality of life, markers of inflammatory response, hospital discharge, health care utilisation, cost and cost effectiveness and adverse events.
Discussion: This is the first trial to examine aortic valve replacement via manubrium-limited ministernotomy versus conventional sternotomy when comparing red blood cell transfusion rates following surgery. Surgical trials present significant challenges; strengths of this trial include a rigorous research design, standardised surgery performed by experienced consultant cardiothoracic surgeons, an agreed anaesthetic regimen, patient blinding and consultant-led patient recruitment. The MAVRIC trial will demonstrate that complex surgical trials can be delivered to exemplary standards and provide the community with the knowledge required to inform future care for patients requiring aortic valve replacement surgery.
Trial registration: International Standard Randomised Controlled Trial Number (ISRCTN) ISRCTN29567910 . Registered on 3 February 2014.
Keywords: Aortic valve replacement (AVR); Inflammatory response; Manubrium-limited ministernotomy; Minimally invasive aortic valve replacement; Red blood cell transfusion; Sternotomy.
Figures
References
-
- O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–42. doi: 10.1016/j.athoracsur.2009.05.056. - DOI - PubMed
-
- The society of cardiothoracic surgery of Great Britain and Ireland. 5th National Adult Cardiac Surgical Database. 2003. www.scts.org/_userfiles/resources/5thBlueBook2003.pdf. Accessed 11 Mar 2016.
-
- Dunning J, Gao H, Chambers J, Moat N, Murphy G, Pagano D, Ray S, Roxburgh J, Bridgewater B. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use — an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National Database. J Thorac Cardiovasc Surg. 2011;142(4):776–82. doi: 10.1016/j.jtcvs.2011.04.048. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources