Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury
- PMID: 28129785
- PMCID: PMC5273809
- DOI: 10.1186/s13287-017-0475-8
Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury
Abstract
Background: Mesenchymal stromal cells (MSCs) represent an option for the treatment of acute kidney injury (AKI). It is known that young stem cells are better than are aged stem cells at reducing the incidence of the senescent phenotype in the kidneys. The objective of this study was to determine whether AKI leads to premature, stress-induced senescence, as well as whether human umbilical cord-derived MSCs (huMSCs) can prevent ischaemia/reperfusion injury (IRI)-induced renal senescence in rats.
Methods: By clamping both renal arteries for 45 min, we induced IRI in male rats. Six hours later, some rats received 1 × 106 huMSCs or human adipose-derived MSCs (aMSCs) intraperitoneally. Rats were euthanised and studied on post-IRI days 2, 7 and 49.
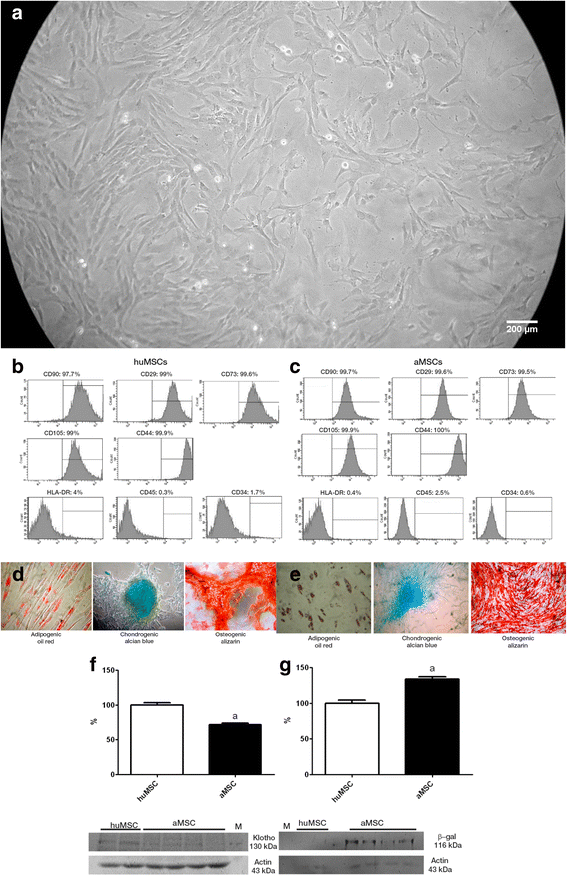
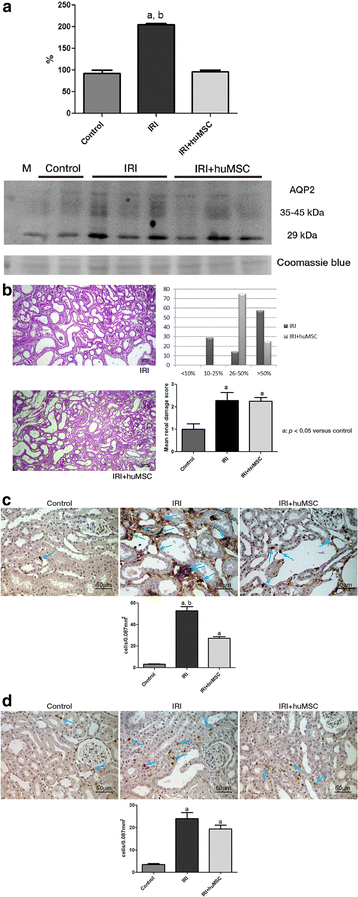
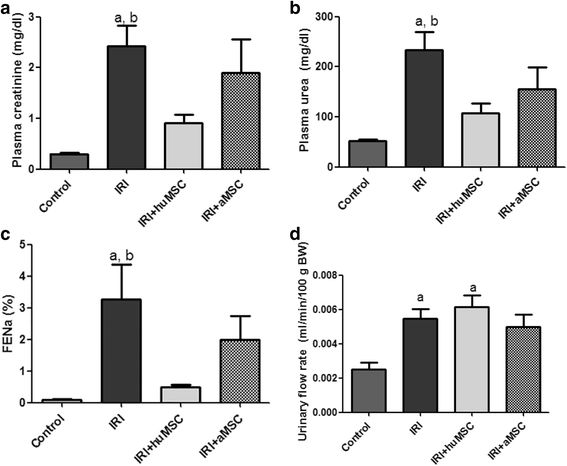
Results: On post-IRI day 2, the kidneys of huMSC-treated rats showed improved glomerular filtration, better tubular function and higher expression of aquaporin 2, as well as less macrophage infiltration. Senescence-related proteins (β-galactosidase, p21Waf1/Cip1, p16INK4a and transforming growth factor beta 1) and microRNAs (miR-29a and miR-34a) were overexpressed after IRI and subsequently downregulated by the treatment. The IRI-induced pro-oxidative state and reduction in Klotho expression were both reversed by the treatment. In comparison with huMSC treatment, the treatment with aMSCs improved renal function to a lesser degree, as well as resulting in a less pronounced increase in the renal expression of Klotho and manganese superoxide dismutase. Treatment with huMSCs ameliorated long-term kidney function after IRI, minimised renal fibrosis, decreased β-galactosidase expression and increased the expression of Klotho.
Conclusions: Our data demonstrate that huMSCs attenuate the inflammatory and oxidative stress responses occurring in AKI, as well as reducing the expression of senescence-related proteins and microRNAs. Our findings broaden perspectives for the treatment of AKI.
Keywords: Acute kidney injury; Mesenchymal stromal cell; Senescence; Telomere; Umbilical cord; microRNA.
Figures







Similar articles
-
The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury.Stem Cell Res Ther. 2017 Feb 7;8(1):24. doi: 10.1186/s13287-017-0478-5. Stem Cell Res Ther. 2017. PMID: 28173878 Free PMC article.
-
Acute Kidney Injury as a Condition of Renal Senescence.Cell Transplant. 2018 May;27(5):739-753. doi: 10.1177/0963689717743512. Epub 2018 Apr 27. Cell Transplant. 2018. PMID: 29701108 Free PMC article. Review.
-
Mesenchymal Stem Cells in Renal Ischemia-Reperfusion Injury: Biological and Therapeutic Perspectives.Curr Stem Cell Res Ther. 2017;12(3):183-187. doi: 10.2174/1574888X11666161024143640. Curr Stem Cell Res Ther. 2017. PMID: 27781940 Review.
-
Klotho gene-modified BMSCs showed elevated antifibrotic effects by inhibiting the Wnt/β-catenin pathway in kidneys after acute injury.Cell Biol Int. 2018 Dec;42(12):1670-1679. doi: 10.1002/cbin.11068. Cell Biol Int. 2018. PMID: 30358003
-
Human umbilical cord mesenchymal stem cells-derived extracellular vesicles ameliorate kidney ischemia-reperfusion injury by suppression of senescent tubular epithelial cells: experimental study.Int J Surg. 2025 Jan 1;111(1):394-410. doi: 10.1097/JS9.0000000000002074. Int J Surg. 2025. PMID: 39236098 Free PMC article.
Cited by
-
Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities.Stem Cell Res Ther. 2021 Mar 3;12(1):161. doi: 10.1186/s13287-021-02212-0. Stem Cell Res Ther. 2021. PMID: 33658073 Free PMC article. Review.
-
Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis.Int J Mol Sci. 2022 Feb 25;23(5):2521. doi: 10.3390/ijms23052521. Int J Mol Sci. 2022. PMID: 35269664 Free PMC article.
-
Current advances of stem cell-based therapy for kidney diseases.World J Stem Cells. 2021 Jul 26;13(7):914-933. doi: 10.4252/wjsc.v13.i7.914. World J Stem Cells. 2021. PMID: 34367484 Free PMC article. Review.
-
The context-dependent effect of cellular senescence: From embryogenesis and wound healing to aging.Ageing Res Rev. 2025 Jul;109:102760. doi: 10.1016/j.arr.2025.102760. Epub 2025 May 1. Ageing Res Rev. 2025. PMID: 40318767 Review.
-
Tetramethylpyrazine enhanced the therapeutic effects of human umbilical cord mesenchymal stem cells in experimental autoimmune encephalomyelitis mice through Nrf2/HO-1 signaling pathway.Stem Cell Res Ther. 2020 May 19;11(1):186. doi: 10.1186/s13287-020-01700-z. Stem Cell Res Ther. 2020. PMID: 32430010 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

