Polymer conjugate of a microtubule destabilizer inhibits lung metastatic melanoma
- PMID: 28130039
- PMCID: PMC5502538
- DOI: 10.1016/j.jconrel.2017.01.028
Polymer conjugate of a microtubule destabilizer inhibits lung metastatic melanoma
Abstract
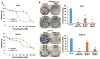
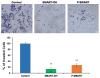
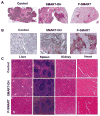
Melanoma is the most aggressive type of skin cancer. It is highly metastatic, migrating through lymph nodes to distant sites of the body, especially to lungs, liver and brain. Systemic chemotherapy remains the mainstay of treatment; however, the development of multidrug resistance (MDR) restricts the efficacy of current chemotherapeutic drugs. We synthesized a series of microtubule destabilizers, substituted methoxybenzoyl-ary-thiazole (SMART) compounds, which inhibited tubulin polymerization and effectively circumvented MDR. Due to poor water solubility of SMART compounds, co-solvent delivery is required for their systemic administration, which is usually associated with hepatotoxicity, nephrotoxicity and hemolysis. To solve this problem and also to increase circulation time, we synthesized a new SMART analogue, SMART-OH, and its polymer-drug conjugate, methoxy-poly (ethylene glycol)-block-poly (2-methyl-2-carboxyl-propylene carbonate-graft-SMART-graft-dodecanol) (abbreviated as P-SMART), with 14.3±2.8% drug payload of SMART-OH. Similar to its parent drug, P-SMART showed significant anticancer activity against melanoma cells in cytotoxicity, colony formation, and cell invasion studies. In addition, P-SMART treatment led to cell cycle arrest at G2/M phase and cell accumulation in sub-G1 phase. We established a model of metastatic melanoma to the lung in C57/BL6 albino mice to determine in vivo efficacy of P-SMART and SMART-OH at the dose of 20mg/kg. P-SMART treatment resulted in significant inhibition of tumor growth and prolonged mouse median survival. In conclusion, P-SMART, a novel polymer-microtubule destabilizer conjugate, has the potential to treat metastatic melanoma.
Keywords: Metastatic melanoma; Microtubule destabilizer; Nanomedicine; Polymer drug conjugate.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: none
Figures





References
-
- Jatzkewitz H. Peptamin (glycyl-L-leucyl-mescaline) bound to blood plasma expander (polyvinylpyrrolidone) as a new depot form of a biologically active primary amine (mescaline) Z Naturforsch. 1955;10:27–31.
-
- Ringsdorf H. Structure and properties of pharmacologically active polymers. 1975;51:135–153.
-
- Coit DG, Andtbacka R, Bichakjian CK, Dilawari RA, Dimaio D, Guild V, Halpern AC, Hodi FS, Kashani-Sabet M, Lange JR, Lind A, Martin L, Martini MC, Pruitt SK, Ross MI, Sener SF, Swetter SM, Tanabe KK, Thompson JA, Trisal V, Urist MM, Weber J, Wong MK. NCCN Melanoma Panel, Melanoma. J Natl Compr Canc Netw. 2009;7:250–275. - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

