Blood culture-PCR to optimise typhoid fever diagnosis after controlled human infection identifies frequent asymptomatic cases and evidence of primary bacteraemia
- PMID: 28130144
- PMCID: PMC5345565
- DOI: 10.1016/j.jinf.2017.01.006
Blood culture-PCR to optimise typhoid fever diagnosis after controlled human infection identifies frequent asymptomatic cases and evidence of primary bacteraemia
Abstract
Background: Improved diagnostics for typhoid are needed; a typhoid controlled human infection model may accelerate their development and translation. Here, we evaluated a blood culture-PCR assay for detecting infection after controlled human infection with S. Typhi and compared test performance with optimally performed blood cultures.
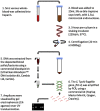
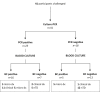
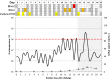
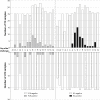
Methodology/principal findings: Culture-PCR amplification of blood samples was performed alongside daily blood culture in 41 participants undergoing typhoid challenge. Study endpoints for typhoid diagnosis (TD) were fever and/or bacteraemia. Overall, 24/41 (59%) participants reached TD, of whom 21/24 (86%) had ≥1 positive blood culture (53/674, 7.9% of all cultures) or 18/24 (75%) had ≥1 positive culture-PCR assay result (57/684, 8.3%). A further five non-bacteraemic participants produced culture-PCR amplicons indicating infection; overall sensitivity/specificity of the assay compared to the study endpoints were 70%/65%. We found no significant difference between blood culture and culture-PCR methods in ability to identify cases (12 mismatching pairs, p = 0.77, binomial test). Clinical and stool culture metadata demonstrated that additional culture-PCR amplification positive individuals likely represented true cases missed by blood culture, suggesting the overall attack rate may be 30/41 (73%) rather than 24/41 (59%). Several participants had positive culture-PCR results soon after ingesting challenge providing new evidence for occurrence of an early primary bacteraemia.
Conclusions/significance: Overall the culture-PCR assay performed well, identifying extra typhoid cases compared with routine blood culture alone. Despite limitations to widespread field-use, the benefits of increased diagnostic yield, reduced blood volume and faster turn-around-time, suggest that this assay could enhance laboratory typhoid diagnostics in research applications and high-incidence settings.
Keywords: Controlled human infection model; Diagnostics; Febrile disease; Polymerase chain reaction; Salmonella Typhi; Typhoid fever.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Parry C.M., Hien T.T., Dougan G., White N.J., Farrar J.J. Typhoid fever. N Engl J Med. 2002;347(22):1770–1782. - PubMed
-
- Darton T.C., Blohmke C.J., Pollard A.J. Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol. 2014;30(1):7–17. - PubMed
-
- Parry C.M., Wijedoru L., Arjyal A., Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther. 2011;9(6):711–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

