Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke
- PMID: 28130211
- PMCID: PMC5399481
- DOI: 10.1182/blood-2016-09-740670
Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke
Abstract
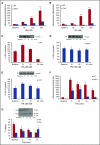
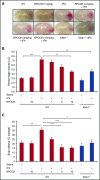
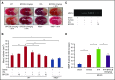
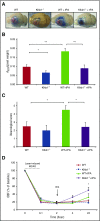
Thrombolytic therapy using tissue plasminogen activator (tPA) in acute stroke is associated with increased risks of cerebral hemorrhagic transformation and angioedema. Although plasma kallikrein (PKal) has been implicated in contributing to both hematoma expansion and thrombosis in stroke, its role in the complications associated with the therapeutic use of tPA in stroke is not yet available. We investigated the effects of tPA on plasma prekallikrein (PPK) activation and the role of PKal on cerebral outcomes in a murine thrombotic stroke model treated with tPA. We show that tPA increases PKal activity in vitro in both murine and human plasma, via a factor XII (FXII)-dependent mechanism. Intravenous administration of tPA increased circulating PKal activity in mice. In mice with thrombotic occlusion of the middle cerebral artery, tPA administration increased brain hemorrhage transformation, infarct volume, and edema. These adverse effects of tPA were ameliorated in PPK (Klkb1)-deficient and FXII-deficient mice and in wild-type (WT) mice pretreated with a PKal inhibitor prior to tPA. tPA-induced brain hemisphere reperfusion after photothrombolic middle cerebral artery occlusion was increased in Klkb1-/- mice compared with WT mice. In addition, PKal inhibition reduced matrix metalloproteinase-9 activity in brain following stroke and tPA therapy. These data demonstrate that tPA activates PPK in plasma and PKal inhibition reduces cerebral complications associated with tPA-mediated thrombolysis in stroke.
© 2017 by The American Society of Hematology.
Figures


Comment in
-
Making thrombolysis safer in stroke.Blood. 2017 Apr 20;129(16):2212-2213. doi: 10.1182/blood-2017-02-765610. Blood. 2017. PMID: 28428237 No abstract available.
References
-
- Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. - PubMed
-
- Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527. - PubMed
-
- Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7(1):59-64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

