Connective Tissue Growth Factor Domain 4 Amplifies Fibrotic Kidney Disease through Activation of LDL Receptor-Related Protein 6
- PMID: 28130402
- PMCID: PMC5461793
- DOI: 10.1681/ASN.2016080826
Connective Tissue Growth Factor Domain 4 Amplifies Fibrotic Kidney Disease through Activation of LDL Receptor-Related Protein 6
Abstract
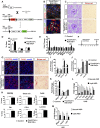
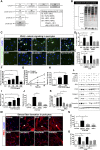
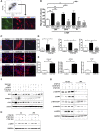
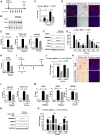
Connective tissue growth factor (CTGF), a matrix-associated protein with four distinct cytokine binding domains, has roles in vasculogenesis, wound healing responses, and fibrogenesis and is upregulated in fibroblasts and myofibroblasts in disease. Here, we investigated the role of CTGF in fibrogenic cells. In mice, tissue-specific inducible overexpression of CTGF by kidney pericytes and fibroblasts had no bearing on nephrogenesis or kidney homeostasis but exacerbated inflammation and fibrosis after ureteral obstruction. These effects required the WNT receptor LDL receptor-related protein 6 (LRP6). Additionally, pericytes isolated from these mice became hypermigratory and hyperproliferative on overexpression of CTGF. CTGF is cleaved in vivo into distinct domains. Treatment with recombinant domain 1, 1+2 (N terminus), or 4 (C terminus) independently activated myofibroblast differentiation and wound healing responses in cultured pericytes, but domain 4 showed the broadest profibrotic activity. Domain 4 exhibited low-affinity binding to LRP6 in in vitro binding assays, and inhibition of LRP6 or critical signaling cascades downstream of LRP6, including JNK and WNT/β-catenin, inhibited the biologic activity of domain 4. Administration of blocking antibodies specifically against CTGF domain 4 or recombinant Dickkopf-related protein-1, an endogenous inhibitor of LRP6, effectively inhibited inflammation and fibrosis associated with ureteral obstruction in vivo Therefore, domain 4 of CTGF and the WNT signaling pathway are important new targets in fibrosis.
Keywords: Connective Tissue Growth Factor; DKK1; LRP6; fibrosis; inflammation; pericytes.
Copyright © 2017 by the American Society of Nephrology.
Figures

References
-
- Lupher ML Jr., Gallatin WM: Regulation of fibrosis by the immune system. Adv Immunol 89: 245–288, 2006 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

