Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration
- PMID: 28130640
- PMCID: PMC5521959
- DOI: 10.1007/s00401-017-1679-9
Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration
Abstract
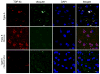
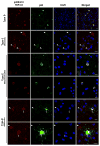
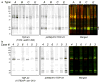
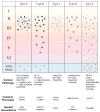
Frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) can typically be categorized into one of four distinct histopathologic patterns of TDP-43 pathology, types A to D. The strength of this histopathologic classification lies in the association between FTLD-TDP subtypes and various clinical and genetic features of disease. Seven cases of FTLD-TDP were identified here which were difficult to classify based on existing pathologic criteria. Distinct features common to these cases included TDP-43 aggregates over a wide neuroanatomic distribution comprised of granulofilamentous neuronal inclusions, abundant grains, and oligodendroglial inclusions. TDP-43 aggregates were phosphorylated and associated with loss of normal nuclear TDP-43 protein (nuclear clearance) but were negative for ubiquitin. Biochemical analysis confirmed the presence of insoluble and phosphorylated TDP-43 and also revealed a distinct pattern of TDP-43 C-terminal fragments relative to other FTLD-TDP subtypes. Finally, these cases were uniformly associated with a very rapid clinical course culminating in death within ~3 years of disease onset. We suggest that these cases may represent a unique clinicopathologic subtype of FTLD-TDP which we provisionally call "type E." The immature appearance of TDP-43 aggregates, widespread distribution, uniform biochemical profile and rapid clinical course highlights the clinical and pathologic variability within FTLD-TDP, and raises the possibility that type E neuropathology is the sequelae of a particularly virulent strain of TDP-43 proteinopathy.
Figures


References
-
- Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Lee EB, Fang L, Van Deerlin VM, Ludolph AC, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. - DOI - PMC - PubMed
-
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

