Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis
- PMID: 28130681
- PMCID: PMC10717645
- DOI: 10.1007/s12576-017-0521-4
Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis
Abstract
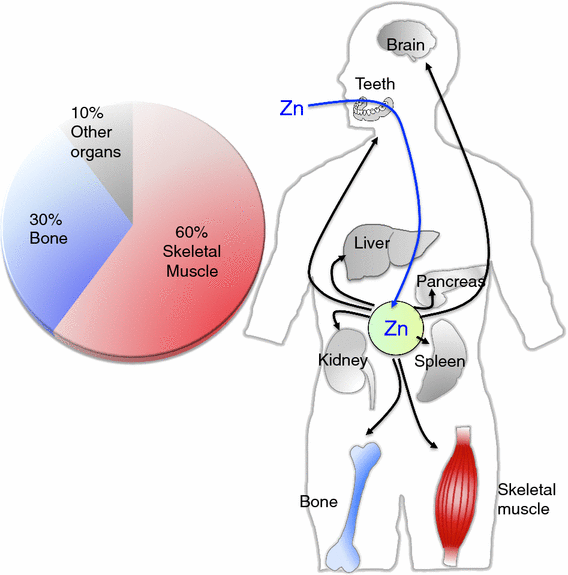
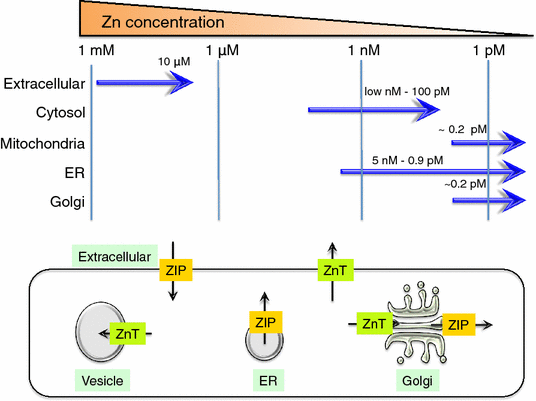
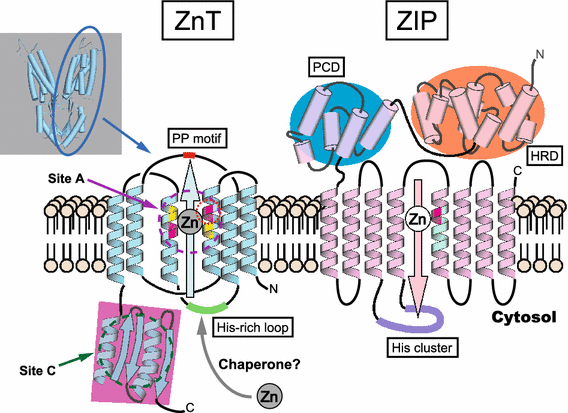
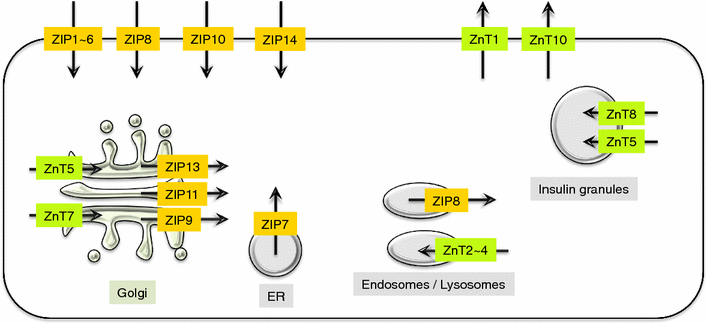
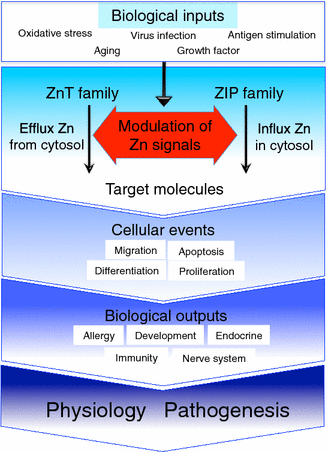
Zinc (Zn) is an essential trace mineral that regulates the expression and activation of biological molecules such as transcription factors, enzymes, adapters, channels, and growth factors, along with their receptors. Zn deficiency or excessive Zn absorption disrupts Zn homeostasis and affects growth, morphogenesis, and immune response, as well as neurosensory and endocrine functions. Zn levels must be adjusted properly to maintain the cellular processes and biological responses necessary for life. Zn transporters regulate Zn levels by controlling Zn influx and efflux between extracellular and intracellular compartments, thus, modulating the Zn concentration and distribution. Although the physiological functions of the Zn transporters remain to be clarified, there is growing evidence that Zn transporters are related to human diseases, and that Zn transporter-mediated Zn ion acts as a signaling factor, called "Zinc signal". Here we describe critical roles of Zn transporters in the body and their contribution at the molecular, biochemical, and genetic levels, and review recently reported disease-related mutations in the Zn transporter genes.
Keywords: Disease; Physiology; Transporter; Zinc; Zinc signaling.
Conflict of interest statement
Author Takafumi Hara declares that he has no conflict of interest. Author Taka-aki Takeda declares that he has no conflict of interest. Author Teruhisa Takagishi declares that he has no conflict of interest. Author Kazuhisa Fukue declares that he has no conflict of interest. Author Taiho Kambe declares that he has no conflict of interest. Author Toshiyuki Fukada declares that he has no conflict of interest.
Figures
References
-
- Prasad AS. Zinc: an overview. Nutr Burbank Los Angel Cty Calif. 1995;11:93–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

