Evaluation of tongue squamous cell carcinoma resection margins using ex-vivo MR
- PMID: 28130702
- PMCID: PMC5420007
- DOI: 10.1007/s11548-017-1524-6
Evaluation of tongue squamous cell carcinoma resection margins using ex-vivo MR
Abstract
Purpose: Purpose of this feasibility study was (1) to evaluate whether application of ex-vivo 7T MR of the resected tongue specimen containing squamous cell carcinoma may provide information on the resection margin status and (2) to evaluate the research and developmental issues that have to be solved for this technique to have the beneficial impact on clinical outcome that we expect: better oncologic and functional outcomes, better quality of life, and lower costs.
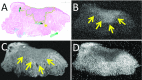
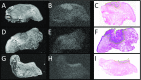
Methods: We performed a non-blinded validation of ex-vivo 7T MR to detect the tongue squamous cell carcinoma and resection margin in 10 fresh tongue specimens using histopathology as gold standard.
Results: In six of seven specimens with a histopathologically determined invasion depth of the tumor of [Formula: see text] mm, the tumor could be recognized on MR, with a resection margin within a 2 mm range as compared to histopathology. In three specimens with an invasion depth of [Formula: see text] mm, the tumor was not visible on MR. Technical limitations mainly included scan time, image resolution, and the fact that we used a less available small-bore 7T MR machine.
Conclusion: Ex-vivo 7T probably will have a low negative predictive value but a high positive predictive value, meaning that in tumors thicker than a few millimeters we expect to be able to predict whether the resection margin is too small. A randomized controlled trial needs to be performed to show our hypothesis: better oncologic and functional outcomes, better quality of life, and lower costs.
Keywords: Ex-vivo; Magnetic resonance imaging; Squamous cell carcinoma; Tongue; Validation.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No external funding was received for this study.
Ethical standards
All procedures were in accordance with the ethical standards of the institutional research committee (reg. 2015/1969) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- Smits RW, Koljenović S, Hardillo JA, Ten Hove I, Meeuwis CA, Sewnaik A, Dronkers EA, Bakker Schut TC, Langeveld TP, Molenaar J, Hegt VN, Puppels GJ, Baatenburg de Jong RJ. Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016;38(Suppl 1):E2197-203. - PubMed
-
- Hinni ML, Ferlito A, Brandwein-Gensler MS, Takes RP, Silver CE, Westra WH, Seethala RR, Rodrigo JP, Corry J, Bradford CR, Hunt JL, Strojan P, Devaney KO, Gnepp DR, Hartl DM, Kowalski LP, Rinaldo A, Barnes L. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35:1362–1370. doi: 10.1002/hed.23110. - DOI - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology, Head and Neck Cancers. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 15 July 2016
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

