Node of Ranvier length as a potential regulator of myelinated axon conduction speed
- PMID: 28130923
- PMCID: PMC5313058
- DOI: 10.7554/eLife.23329
Node of Ranvier length as a potential regulator of myelinated axon conduction speed
Abstract
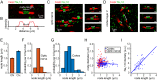
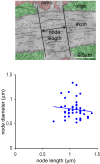
Myelination speeds conduction of the nerve impulse, enhancing cognitive power. Changes of white matter structure contribute to learning, and are often assumed to reflect an altered number of myelin wraps. We now show that, in rat optic nerve and cerebral cortical axons, the node of Ranvier length varies over a 4.4-fold and 8.7-fold range respectively and that variation of the node length is much less along axons than between axons. Modelling predicts that these node length differences will alter conduction speed by ~20%, similar to the changes produced by altering the number of myelin wraps or the internode length. For a given change of conduction speed, the membrane area change needed at the node is >270-fold less than that needed in the myelin sheath. Thus, axon-specific adjustment of node of Ranvier length is potentially an energy-efficient and rapid mechanism for tuning the arrival time of information in the CNS.
Keywords: axon; conduction speed; myelin; neuroscience; node of Ranvier; rat.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

