A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers
- PMID: 28131890
- PMCID: PMC5562927
- DOI: 10.1016/j.neuroimage.2017.01.034
A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers
Abstract
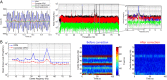
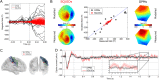
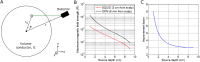
Advances in the field of quantum sensing mean that magnetic field sensors, operating at room temperature, are now able to achieve sensitivity similar to that of cryogenically cooled devices (SQUIDs). This means that room temperature magnetoencephalography (MEG), with a greatly increased flexibility of sensor placement can now be considered. Further, these new sensors can be placed directly on the scalp surface giving, theoretically, a large increase in the magnitude of the measured signal. Here, we present recordings made using a single optically-pumped magnetometer (OPM) in combination with a 3D-printed head-cast designed to accurately locate and orient the sensor relative to brain anatomy. Since our OPM is configured as a magnetometer it is highly sensitive to environmental interference. However, we show that this problem can be ameliorated via the use of simultaneous reference sensor recordings. Using median nerve stimulation, we show that the OPM can detect both evoked (phase-locked) and induced (non-phase-locked oscillatory) changes when placed over sensory cortex, with signals ~4 times larger than equivalent SQUID measurements. Using source modelling, we show that our system allows localisation of the evoked response to somatosensory cortex. Further, source-space modelling shows that, with 13 sequential OPM measurements, source-space signal-to-noise ratio (SNR) is comparable to that from a 271-channel SQUID system. Our results highlight the opportunity presented by OPMs to generate uncooled, potentially low-cost, high SNR MEG systems.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Brookes M.J., Vrba J., Robinson S.E., Stevenson C.M., Peters A.M., Barnes G.R., Hillebrand A., Morris P.G. Optimising experimental design for MEG beamformer imaging. NeuroImage. 2008;39:1788–1802. - PubMed
-
- Cheyne D.O. MEG studies of sensorimotor rhythms: a review. Exp. Neurol. 2013;245:27–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

