Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, αCT1, reduces VEGF-dependent RPE pathophysiology
- PMID: 28132078
- PMCID: PMC5710814
- DOI: 10.1007/s00109-017-1506-8
Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, αCT1, reduces VEGF-dependent RPE pathophysiology
Abstract
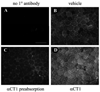
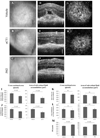
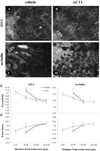
A critical target tissue in age-related macular degeneration (AMD) is the retinal pigment epithelium (RPE), which forms the outer blood-retina barrier (BRB). RPE-barrier dysfunction might result from attenuation/disruption of intercellular tight junctions. Zonula occludens-1 (ZO-1) is a major structural protein of intercellular junctions. A connexin43-based peptide mimetic, αCT1, was developed to competitively block interactions at the PDZ2 domain of ZO-1, thereby inhibiting ligands that selectively bind to this domain. We hypothesized that targeting ZO-1 signaling using αCT1 would maintain BRB integrity and reduce RPE pathophysiology by stabilizing gap- and/or tight-junctions. RPE-cell barrier dysfunction was generated in mice using laser photocoagulation triggering choroidal neovascularization (CNV) or bright light exposure leading to morphological damage. αCT1 was delivered via eye drops. αCT1 treatment reduced CNV development and fluid leakage as determined by optical coherence tomography, and damage was correlated with disruption in cellular integrity of surrounding RPE cells. Light damage significantly disrupted RPE cell morphology as determined by ZO-1 and occludin staining and tiling pattern analysis, which was prevented by αCT1 pre-treatment. In vitro experiments using RPE and MDCK monolayers indicated that αCT1 stabilizes tight junctions, independent of its effects on Cx43. Taken together, stabilization of intercellular junctions by αCT1 was effective in ameliorating RPE dysfunction in models of AMD-like pathology.
Key message: The connexin43 mimetic αCT1 accumulates in the mouse retinal pigment epithelium following topical delivery via eye drops. αCT1 eye drops prevented RPE-cell barrier dysfunction in two mouse models. αCT1 stabilizes intercellular tight junctions. Stabilization of cellular junctions via αCT1 may serve as a novel therapeutic approach for both wet and dry age-related macular degeneration.
Keywords: Age-related macular degeneration; Choroidal neovascularization; Connexin43; Light damage; Retinal pigment epithelium; Tight junctions; Vascular endothelial growth factor.
Figures



Similar articles
-
The Cx43 Carboxyl-Terminal Mimetic Peptide αCT1 Protects Endothelial Barrier Function in a ZO1 Binding-Competent Manner.Biomolecules. 2021 Aug 12;11(8):1192. doi: 10.3390/biom11081192. Biomolecules. 2021. PMID: 34439858 Free PMC article.
-
Retinal Pigment Epithelium Remodeling in Mouse Models of Retinitis Pigmentosa.Int J Mol Sci. 2021 May 20;22(10):5381. doi: 10.3390/ijms22105381. Int J Mol Sci. 2021. PMID: 34065385 Free PMC article.
-
Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice.Neurobiol Aging. 2014 Sep;35(9):2013-20. doi: 10.1016/j.neurobiolaging.2014.03.008. Epub 2014 Mar 15. Neurobiol Aging. 2014. PMID: 24709310
-
Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions.Ann N Y Acad Sci. 2009 May;1165:113-20. doi: 10.1111/j.1749-6632.2009.04440.x. Ann N Y Acad Sci. 2009. PMID: 19538295 Free PMC article. Review.
-
Key Claudins at the Blood-Retina Barriers.Adv Exp Med Biol. 2025;1468:447-451. doi: 10.1007/978-3-031-76550-6_73. Adv Exp Med Biol. 2025. PMID: 39930236 Review.
Cited by
-
Carnosine Counteracts the Molecular Alterations Aβ Oligomers-Induced in Human Retinal Pigment Epithelial Cells.Molecules. 2023 Apr 9;28(8):3324. doi: 10.3390/molecules28083324. Molecules. 2023. PMID: 37110558 Free PMC article.
-
Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations.Biology (Basel). 2024 Apr 6;13(4):243. doi: 10.3390/biology13040243. Biology (Basel). 2024. PMID: 38666855 Free PMC article. Review.
-
Retinal G-protein-coupled receptor deletion exacerbates AMD-like changes via the PINK1-parkin pathway under oxidative stress.FASEB J. 2024 Oct;38(20):e70135. doi: 10.1096/fj.202401160RR. FASEB J. 2024. PMID: 39467145 Free PMC article.
-
Claudin-1 silencing increases sensitivity of liver cancer HepG2 cells to 5-fluorouracil by inhibiting autophagy.Oncol Lett. 2019 Dec;18(6):5709-5716. doi: 10.3892/ol.2019.10967. Epub 2019 Oct 8. Oncol Lett. 2019. PMID: 31788043 Free PMC article.
-
Cx43 and the Actin Cytoskeleton: Novel Roles and Implications for Cell-Cell Junction-Based Barrier Function Regulation.Biomolecules. 2020 Dec 10;10(12):1656. doi: 10.3390/biom10121656. Biomolecules. 2020. PMID: 33321985 Free PMC article. Review.
References
-
- Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Canadian Journal of Opthalmology. 2005;40:277–287. - PubMed
-
- Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. - PubMed
-
- Hageman Gregory S, Luthertb Phil J, Victor Chong NH, Johnsonc Lincoln V, Don Andersonc H, Mullinsa RF. An Integrated Hypothesis That Considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch’s Membrane Interface in Aging and Age-Related Macular Degeneration. Progress in Retinal and Eye Research. 2001;20:705–732. - PubMed
-
- Strauss O. The Retinal Pigment Epithelium in Visual Function. Physiological Reviews. 2005;85:845–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

