Cardiac Light Chain Amyloidosis: The Role of Metal Ions in Oxidative Stress and Mitochondrial Damage
- PMID: 28132512
- PMCID: PMC5567464
- DOI: 10.1089/ars.2016.6848
Cardiac Light Chain Amyloidosis: The Role of Metal Ions in Oxidative Stress and Mitochondrial Damage
Abstract
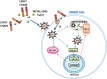
Aims: The knowledge of the mechanism underlying the cardiac damage in immunoglobulin light chain (LC) amyloidosis (AL) is essential to develop novel therapies and improve patients' outcome. Although an active role of reactive oxygen species (ROS) in LC-induced cardiotoxicity has already been envisaged, the actual mechanisms behind their generation remain elusive. This study was aimed at further dissecting the action of ROS generated by cardiotoxic LC in vivo and investigating whether transition metal ions are involved in this process. In the absence of reliable vertebrate model of AL, we used the nematode Caenorhabditis elegans, whose pharynx is an "ancestral heart."
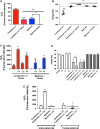
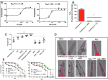
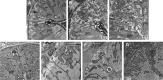
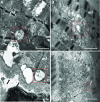
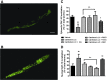
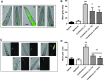
Results: LC purified from patients with severe cardiac involvement intrinsically generated high levels of ROS and when administered to C. elegans induced ROS production, activation of the DAF-16/forkhead transcription factor (FOXO) pathway, and expression of proteins involved in stress resistance and survival. Profound functional and structural ROS-mediated mitochondrial damage, similar to that observed in amyloid-affected hearts from AL patients, was observed. All these effects were entirely dependent on the presence of metal ions since addition of metal chelator or metal-binding 8-hydroxyquinoline compounds (chelex, PBT2, and clioquinol) permanently blocked the ROS production and prevented the cardiotoxic effects of amyloid LC. Innovation and Conclusion: Our findings identify the key role of metal ions in driving the ROS-mediated toxic effects of LC. This is a novel conceptual advance that paves the way for new pharmacological strategies aimed at not only counteracting but also totally inhibiting the vicious cycle of redox damage. Antioxid. Redox Signal. 27, 567-582.
Keywords: amyloid; caenorhabditis elegans; immunoglobulin light chain; metals; mitochondria; protein misfolding.
Conflict of interest statement
R.A.C. is a paid consultant and a shareholder in Prana Biotechnology Ltd. The other authors have no competing interests.
Figures

References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, and Bush AI. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 59: 43–55, 2008 - PubMed
-
- Avery L. and Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron 3: 473–485, 1989 - PubMed
-
- Barnham KJ. and Bush AI. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43: 6727–6749, 2014 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

