Direct Intracranial Injection of AAVrh8 Encoding Monkey β-N-Acetylhexosaminidase Causes Neurotoxicity in the Primate Brain
- PMID: 28132521
- PMCID: PMC5488349
- DOI: 10.1089/hum.2016.109
Direct Intracranial Injection of AAVrh8 Encoding Monkey β-N-Acetylhexosaminidase Causes Neurotoxicity in the Primate Brain
Abstract
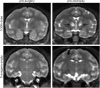
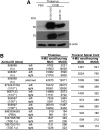
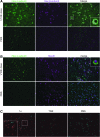
GM2 gangliosidoses, including Tay-Sachs disease and Sandhoff disease, are lysosomal storage disorders caused by deficiencies in β-N-acetylhexosaminidase (Hex). Patients are afflicted primarily with progressive central nervous system (CNS) dysfunction. Studies in mice, cats, and sheep have indicated safety and widespread distribution of Hex in the CNS after intracranial vector infusion of AAVrh8 vectors encoding species-specific Hex α- or β-subunits at a 1:1 ratio. Here, a safety study was conducted in cynomolgus macaques (cm), modeling previous animal studies, with bilateral infusion in the thalamus as well as in left lateral ventricle of AAVrh8 vectors encoding cm Hex α- and β-subunits. Three doses (3.2 × 1012 vg [n = 3]; 3.2 × 1011 vg [n = 2]; or 1.1 × 1011 vg [n = 2]) were tested, with controls infused with vehicle (n = 1) or transgene empty AAVrh8 vector at the highest dose (n = 2). Most monkeys receiving AAVrh8-cmHexα/β developed dyskinesias, ataxia, and loss of dexterity, with higher dose animals eventually becoming apathetic. Time to onset of symptoms was dose dependent, with the highest-dose cohort producing symptoms within a month of infusion. One monkey in the lowest-dose cohort was behaviorally asymptomatic but had magnetic resonance imaging abnormalities in the thalami. Histopathology was similar in all monkeys injected with AAVrh8-cmHexα/β, showing severe white and gray matter necrosis along the injection track, reactive vasculature, and the presence of neurons with granular eosinophilic material. Lesions were minimal to absent in both control cohorts. Despite cellular loss, a dramatic increase in Hex activity was measured in the thalamus, and none of the animals presented with antibody titers against Hex. The high overexpression of Hex protein is likely to blame for this negative outcome, and this study demonstrates the variations in safety profiles of AAVrh8-Hexα/β intracranial injection among different species, despite encoding for self-proteins.
Keywords: AAV; Tay-Sachs disease; adeno-associated virus; gene therapy; hexosaminidase; intracranial delivery.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Gravel RA, Kaback MM, Proia RL, et al. . The GM2 gangliosidoses. In: Scriver C, Beaudet AL, Sly WS, Valle D, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill, 2001
-
- Desnick RJ, Schuchman EH. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu Rev Genomics Hum Genet 2012;13:307–335 - PubMed
-
- Johnson WG, Desnick RJ, Long DM, et al. . Intravenous injection of purified hexosaminidase A into a patient with Tay–Sachs disease. Birth Defects Orig Artic Ser 1973;9:120–124 - PubMed
-
- Enns GM, Huhn SL. Central nervous system therapy for lysosomal storage disorders. Neurosurg Focus 2008;24:E12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

