Reducing non-injection drug use in HIV primary care: A randomized trial of brief motivational interviewing, with and without HealthCall, a technology-based enhancement
- PMID: 28132704
- PMCID: PMC5931702
- DOI: 10.1016/j.jsat.2016.12.009
Reducing non-injection drug use in HIV primary care: A randomized trial of brief motivational interviewing, with and without HealthCall, a technology-based enhancement
Abstract
Aims: In HIV-infected individuals, non-injection drug use (NIDU) compromises many health outcomes. In HIV primary care, the efficacy of brief motivational interviewing (MI) to reduce NIDU is unknown, and drug users may need greater intervention. We designed an enhancement to MI, HealthCall (HC), for daily patient self-monitoring calls to an interactive voice response (IVR) phone system, and provided participants with periodic personalized feedback. To reduce NIDU among HIV primary care patients, we compared the efficacy of MI+HealthCall to MI-only and an educational control condition.
Design: Participants age >18 with >4days of NIDU during the prior 30days were recruited from large urban HIV primary care clinics. Of the 240 participants, 83 were randomly assigned to control, 77 to MI-only, and 80 to MI+HC. Counselors provided educational control, MI-only or MI+HC at baseline. At 30 and 60days (end-of-treatment), counselors briefly discussed drug use, moods and health behaviors, using HealthCall-generated graphs with MI+HC patients. Primary outcomes (last 30days) were number of days used primary drug (NumDU), and total quantity of primary drug used (dollar amount spent; QuantU), derived from the Time-Line Follow-Back.
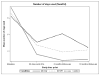
Findings: Across all groups, at end-of-treatment, frequency and quantity of NIDU decreased, with significantly greater reductions in the MI-Only group. A twelve-month post-treatment follow-up indicated sustained benefits of MI+HC and MI-only relative to control.
Conclusions: Brief interventions can be successfully used to reduce non-injection drug use in HIV primary care. IVR-based technology may not be sufficiently engaging to be effective. Future studies should investigate mobile technology to deliver a more engaging version of HealthCall to diverse substance abusing populations.
Keywords: Drug use; HIV; IVR intervention; Motivational interviewing.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
None.
Figures



References
-
- Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases. 2004;39(5):609–629. - PubMed
-
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of Acquired Immune Deficiency Syndromes. 2009;50(1):93–99. - PubMed
-
- Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosomatic Medicine. 2007;69(8):785–792. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

